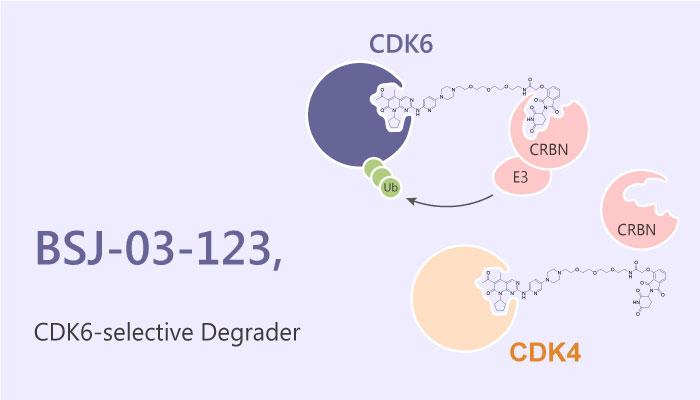
A study from Matthias Brand reported a novel phthalimide-based degrader BSJ-03-123. This compound exploits protein-interface determinants to achieve proteome-wide selectivity for the degradation of cyclin-dependent kinase 6 (CDK6). Notably, CDK6 degradation targets a selective dependency of acute myeloid leukemia cells. Cyclin-dependent kinases (CDKs) participate in regulating fundamental cellular processes such as cell cycle and transcription. CDKs are serine/threonine kinases and their activity depends on binding with cyclins, resulting in temporal control over enzymatic function.
The authors described the discovery and characterization of BSJ-03-123 (BSJ) as a selective CDK6 degradder. In vitro, BSJ-03-123 induced CRBN-dependent and homolog-selective degradation of CDK6. And CDK6 was the only destabilized protein. In addition, BSJ-03-123 induced dose-dependent ternary complex formation with CDK6 and CRBN, but not with CDK4 and CRBN. Thus, it can recognize the structural difference between CDK4 and CDK6, showing excellent selectivity.
Furthermore, BSJ-03-123 caused significantly inhibition of AML cells anti-proliferation by inducing a G1 cell-cycle arrest without a measurable increase in apoptosis. And the inhibition may be due to the disruption of additional, kinase activity-independent molecular mechanisms, or due to pharmacologic advantages of degraders, such as catalytic target turnover. Then, the authors also identified the effect of BSJ-03-123 on the phosphorylation site S780 of CDK4/6. Of note, it can reduce levels of p-S780 significantly.
Reference:
Cell Chem Biol. 2019 Feb 21;26(2):300-306.e9.