The Ras superfamily of low molecular weight GDP/GTP-binding GTPases is critical in the regulation of many important biological events. They include the cell division cycle, survival and death, differentiation, and development. These GTPases also show a relation with oncogenesis, Ras and Rho proteins mediate malignant transformation, invasion, and metastasis. These proteins require prenylation, a lipid posttranslational modification, for biological function. Some proteins such as H-Ras, N-Ras, and K-Ras are farnesylated, and others such as RhoA, Rap1, and R-Ras are geranylgeranylated, whereas RhoB is both farnesylated and geranylgeranylated. Interestingly, K-Ras, possibly N-Ras, but not H-Ras, can become geranylgeranylated if protein farnesylation is inhibited. The two enzymes responsible for this prenylation are farnesyltransferase (FTase) and geranylgeranyltransferase I (GGTase I). FTase prefers to farnesylate proteins in which X is methionine or serine, whereas GGTase I geranylgeranylates proteins in which X is leucine. GGTI-2154 is a potent and selective inhibitor of GGTase I.
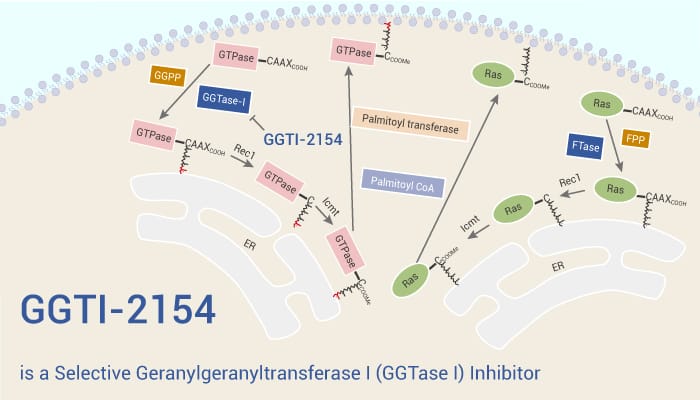
GGTI-2154 inhibits the transfer of geranylgeranyl from [3H]GGPP to H-Ras CVLL. The IC50 is 21 nM. GGTI-2154 (100 mg/kg/day; s.c. for 14 days) induces breast tumor regression in MMTV-ν-Ha-Ras transgenic mice. GGTI-2154 (50 mg/kg/day; i.p. for 50 day) inhibits A-549 tumor growth in nude mice by 60%.Combination therapy of GGTI-2154 with cisplatin, gemcitabine, or Taxol is also more effective. Finally, FTI-2148 and GGTI-2154 are 30- and 33-fold more selective and 30- and 16-fold more potent in whole cells than thiol-containing FTI-276 and GGTI-297, respectively.
In summary, GGTI-2154 is a potent and selective inhibitor of GGTase I, with an IC50 of 21 nM. GGTI-2154 shows more than 200-fold selectivity for GGTase I over FTase. GGTI-2154 has the potential for the research of cancer.
Reference:
Sun J, et, al. Cancer Res. 1999 Oct 1;59(19):4919-26.