Hsp90 (heat shock protein 90) is a molecular chaperone protein. Specifically, Hsp90 can help other proteins fold correctly, stabilize proteins, resist heat stress, and contribute to protein degradation. It can also stabilize many proteins needed for tumor growth. Besides, Hsp90 is present in all branches of bacteria and eukaryotes. Hsp90 is also a mediator of cell homeostasis. Moreover, it promotes many transient low-affinity protein-protein interactions. Hsp90 is a conformational dynamic protein.
Furthermore, Hsp90 is a highly abundant and ubiquitous molecular chaperone. It plays an important role in many cellular processes, including cell cycle control, cell survival, hormones, and other signaling pathways. Meanwhile, it is important for cell response to stress and is a key factor in maintaining cell homeostasis. Many Hsp90 inhibitors is targeting ATP binding sites or carboxyl-terminal domains. Nonetheless, Hsp90 is relevant to many diseases, such as cancer, neurodegenerative diseases, and infectious diseases caused by viruses and protozoa. Inhibition of Hsp90 will change the Hsp90 client protein complex. Importantly, this results in reduced activity, misfolding, ubiquitination, and ultimately proteasome degradation of the client protein. Today, we will introduce a near-infrared dye tethered Hsp90 inhibitor, HS-131.
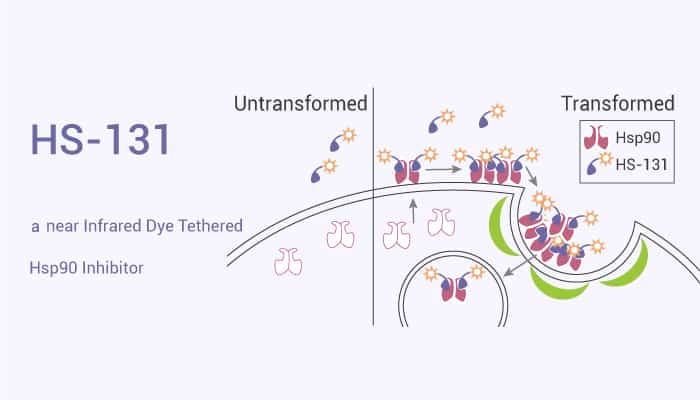
HS-131 is a near Infrared Dye Tethered Hsp90 Inhibitor.
To begin with, HS-131 is able to detect oncogene-driven breast cancers, including multiple different molecular subtypes of human breast cancers. Particularly, HS131 imaging is sensitive and specific in detecting 4T1 breast cancer cell lines and subclones with different metastatic potentials.
Next in importance, HS131 detects spontaneous tumors in MMTV-neu mice and primary and metastatic human breast cancer xenografts. Obviously, HS131 can image invasive lobular breast cancer.
Again, HS131 with 10 nmoL/mouse by iv could label breast cancers with different molecular subtypes. Interestingly, It includes MCF-7 and T-47D for luminal type, BT474M1 and KPL-4 for HER2+ subtype. All breast cancer xenografts with different molecular subtypes showed stronger nIR signals in tumors by intravenous administration of HS131 compared to HS152. Additionally, the retention of the nIR signals was detectable even at the 24 h time point in HS131 injected mice.
References:
Takuya Osada, et al. Clin Cancer Res. 2017 Dec 15;23(24):7531-7542.