CRISPR interference (CRISPRi) is a genetic perturbation technique that allows for sequence-specific repression of gene expression in prokaryotic and eukaryotic cells. In particular, CRISPRi is an RNA-based method for targeted silencing of transcription in bacteria and human cells. The CRISPRi system is derived from the Streptococcus pyogenes CRISPR pathway. Gratifyingly, CRISPRi system requires only the coexpression of a catalytically inactive Cas9 protein and a customizable single guide RNA (sgRNA). Moreover, the Cas9-sgRNA complex binds to DNA elements complementary to the sgRNA. Besides, Cas9-sgRNA complex also causes a steric block that halts transcript elongation by RNA polymerase.
Recently, breast cancer cells utilize the chemokine receptor CX3CR1 to exit the blood circulation. Especially, chemokines are essential for the function of the immune system. Moreover, Chemokines bind to seven transmembrane G protein-coupled receptors that trigger intracellular signaling that drives cell polarization, adhesion, and migration. Chemokines divide into four families based upon structure: CXC, CC, CX3C, and C chemokines. Of these, CX3C chemokine receptor 1 (CX3CR1; GPR13) is a protein that in humans is encoded by the CX3CR1 gene.
In this study, building on the target validation studies, Fei Shen, et al synthesized JMS-17-2. Firstly, JMS-17-2 is a CX3CR1 antagonist. Crucially, JMS-17-2 potently antagonizes CX3CR1 signaling in a dose-dependent fashion, as measured by inhibition of ERK phosphorylation. In addition, the two concentrations of JMS-17-2 most effective in blocking FKN-induced ERK phosphorylation also significantly reduce the migration of breast cancer cells in vitro.
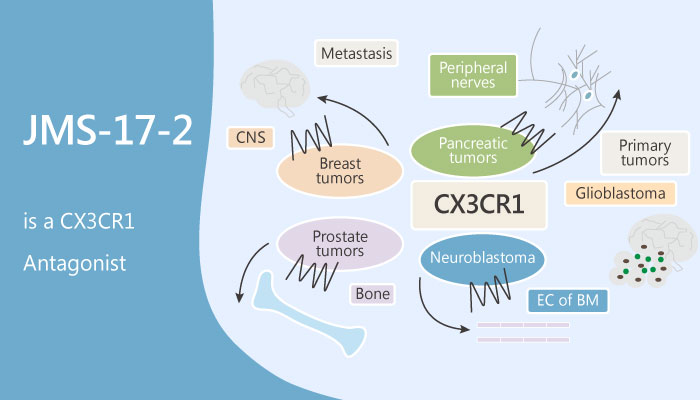
All in all, JMS-17-2 is a potent and selective small-molecule antagonist of CX3CR1, for models of seeding and established metastasis.