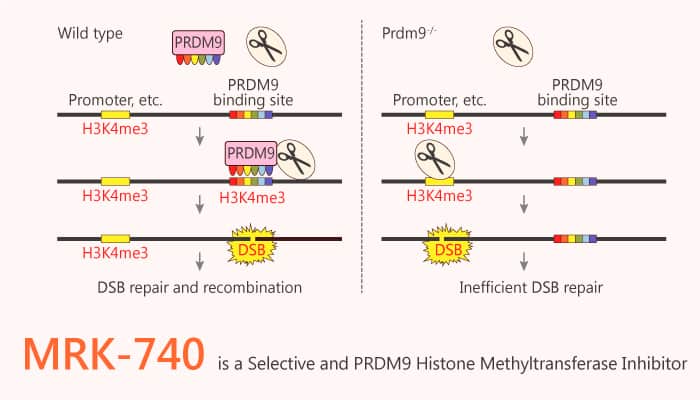
PRDM9 is a zinc finger protein that binds DNA at specific locations in the genome and alleles influence human chromosomal rearrangements, causing hereditary diseases. MRK-740 is a potent selective and cell-active PRDM9 inhibitor with an IC50 of 80 nM.
In cells, MRK-740 specifically and directly inhibits H3K4 methylation at endogenous PRDM9 target loci. Moreover, it binds in the substrate-binding pocket. The discovery of MRK-740 as a chemical probe for the PRDM subfamily of methyltransferases highlights the potential for exploiting SAM in targeting SAM-dependent methyltransferases. Using the enzyme activity assay, MRK-740 is selective against 32 protein, DNA and RNA methyltransferases and PRDM7.
MRK-740 inhibits H3K4 trimethylation by PRDM9 in cells. Furthermore, it reduces PRDM9-dependent trimethylation of ectopic H3K4 (IC50=0.8 µM) and endogenous H3K4 in a concentration-dependent manner. Importantly, MRK-740 is an equipotent inhibitor of H3K4 methylation in MCF7 cells. Furthermore, 10 µM exhibits minimal impact on MCF7 cell viability in a 5-day proliferation assay.
In this study, researchers directly inhibit PRDM9 catalytic activity on chromatin, reducing H3K4me3 levels at intragenic and intergenic target sites. These results indicate that MRK-740 is a potent and cell-active PRDM9 inhibitor that together with the negative control compound MRK-740-NC, can serve as a useful tool for studying the physiological and pathological functions of PRDM9. MRK-740 specifically inhibits methylation on endogenous chromatin, but does not affect H3K4 methylation activity. Interestingly, MRK-740 also inhibits PRDM7 with an IC50 of 45 µM. A first-in-class PRDM inhibitor, it binds to PRDM9 in a SAM-dependent manner with an unusually large SAM interaction interface.
All in all, MRK-740 is a first-in-class chemical probe for PRDM9 that selectively inhibits its methyltransferase activity in biochemical and cellular assays.