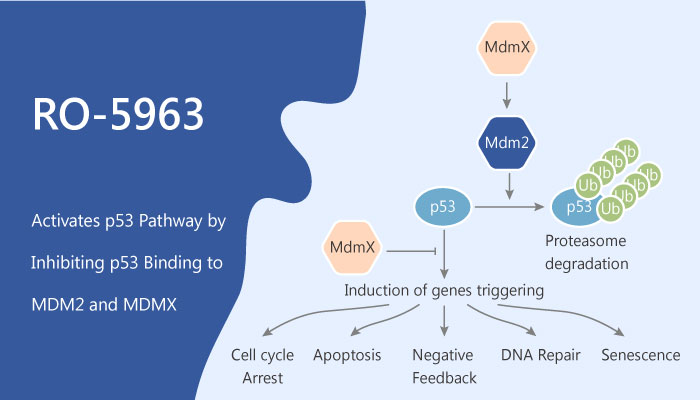
The tumor suppressor p53 is a powerful growth-suppressive and proapoptotic protein. Murine double minute (MDM) 2 and MDMX, as its negative regulators, tightly controlls p53. These proteins bind p53 with their structurally similar N-terminal domains. Therefore, they effectively inhibit p53 transcriptional activity. But, it is only functional in MDM2, which serves as a specific E3 ligase and main regulator of p53 stability. MDMX does not have an intrinsic ligase activity and does not affect directly p53 stability. However, MDMX can enhance ligase activity of MDM2 toward p53 by forming MDM2/MDMX heterodimers. Thus, restoring p53 function via antagonizing the binding of MDM2 and MDMX to p53 is a potential strategy. on the other hand, high levels of MDMX protein can make MDM2 antagonists ineffective in killing cancer cells. Thus, it is necessary to inhibit MDM2 and MDMX simultaneously to release the full activity of stabilized p53.
Recent efforts have been focused on identification of dual MDM2/MDMX antagonists.
In this study, RO-5963 is a dual p53-MDM2 and p53-MDMX inhibitor. Meanwhile, RO-5963 exerts IC50s of ~17 nM and ~24 nM for p53-MDM2 and p53-MDMX, respectively. RO-5963 induces protein dimerization and effectively restore p53 activity in MDMX-overexpressing cancer cells.
RO-5963 penetrates MDMX-overexpressing breast cancer cells (MCF7), stabilizes p53, and elevated protein levels of p21 and MDM2 in a dose-dependent manner. In addition, RO-5963 shows much higher apoptotic activity than Nutlin in both MCF7 and ZR75-30 cell lines.
In summary, RO-5963 can restore p53 apoptotic activity in the presence of high levels of MDMX. It may offer a more effective therapeutic modality for MDMX-overexpressing cancers.
Reference: