Microtubule, a highly dynamic structure, forms spindle fibers during mitosis. Several key cellular processes such as vesicular trafficking, intracellular signalling, cell motility and mitotic cell division depend on microtubules. In recent years, emerging studies have demonstrated the role of microtubule in targeted cancer therapy. For example, Paclitaxel, the most widely applied drug in cancer treatment, shows potent anticancer activity in various cancers, including ovarian cancer, breast cancer, lung cancer, Kaposi sarcoma, cervical cancer, and pancreatic cancer. The anti-cancer drug has been approved for medical use by Food and Drug Administration in 1993 and is also on the World Health Organization’s List of Essential Medicines.
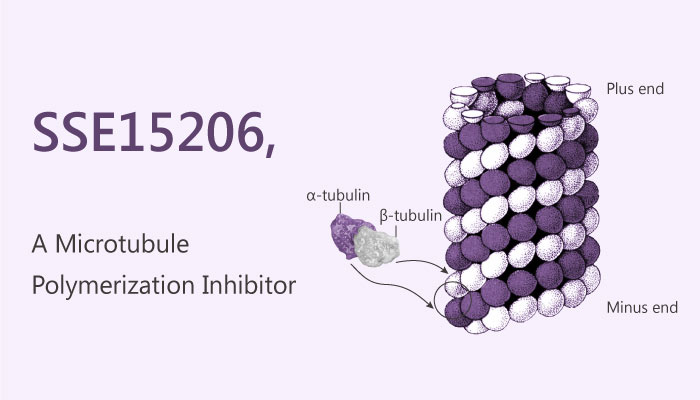
A study from Safia Manzoor, et al. described a novel microtubule polymerization inhibitor, SSE15206. This inhibitor is a pyrazolinethioamide derivative that has potent antiproliferative activities in cancer cell lines of different origins and overcomes resistance to microtubule-targeting agents. SSE15206 is a microtubule polymerization inhibitor, with a GI50 of 197 nM in a SRB proliferation assay in HCT116 cells. In the study, it also caused aberrant mitosis resulting in G2/M arrest due to incomplete spindle formation in cancer cells. In addition, SSE15206 treatment with concentration of 0.16-2.5 μM in HCT116 cells increased phosphorylation of histone H3 and MPM2 dese-dependently. Not only that, SSE15206 also induced cleaved PARP and p53 in HCT116, A549, and CAL-51 cells.
Notably, acquisition of resistance to one type of drug often causes resistance to several drug types or “multidrug resistance”, especially for anti-microtubule drugs. More importantly, in the study, the authors demonstrated that SSE15206 was able to overcome the resistance of different cancer cell lines to chemotherapy drugs, including multidrug-resistant kB-V1 and A2780-Pac-Res cell lines overexpressing MDR-1. Hopefully, SSE15206 may be a promising hit for the lead optimization study of targeted multidrug resistance.