Protein kinases inhibition is a common therapeutic strategy for many diseases, including cancers, inflammatory diseases, diabetes and neurodegenerative disorders. The Ser-/Thr-specific kinase Akt is also known as protein kinase B. It plays a key role in PKBPI3K/Akt/mTOR-pathway. Alterations and disorders in the PI3K/Akt signaling pathway relate to different types of solid tumors such as lung cancer, and melanoma. Emerge evidences have identified Akt inhibitors as common cancer drugs in recent decades. A study from Niklas Uhlenbrock, et al. discovered and identified an Akt inhibitor Borussertib. It displays covalent-allosteric binding characterizaton.
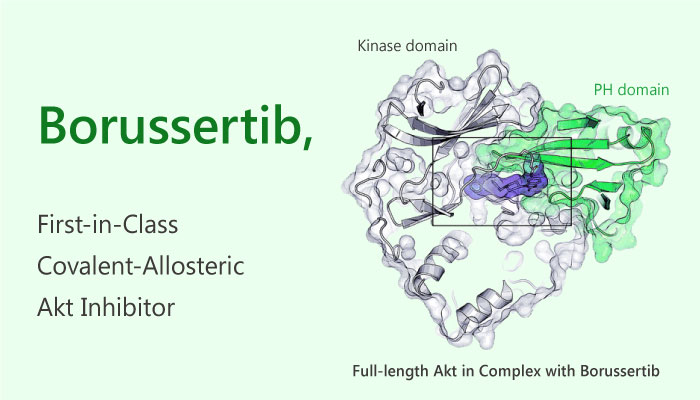
Firstly in structure, Borussertib bound between the PH- and the kinase domain to adopt the proposed allosteric binding mode. The complex of Borussertib and Akt irreversibly stabilized the inactive PH-in conformation.
In biochemical assays, Borussertib showed an IC50 of 0.8 nM and a Ki of 2.2 nM for Aktwt. Furthermore, in cell assays, the inhibitor exhibited excellent cellular activity in the nanomolar range. The EC50 values are 191±90 nM, 48±15 nM, 5±1 nM, 277±90 nM, 373±54 nM, 7770±641 nM in AN3CA (endometrium), T47D (breast), ZR-75-1 (breast), MCF-7 (breast), BT-474 (breast) and KU-19-19 (bladder) cell lines, respectively.
In addition, this compound also revealed a good pharmacokinetic profile with reasonable microsomal stability in human and murine microsomes. Besides, based on the crystal structure of Borussertib disclosed firstly, the authors designed several other compounds with good efficacy and pharmacokinetic profiles.
The structural information emanating from the recently published first crystal structure of a covalent-allosteric Akt inhibitor Borussertib inspired a series of structure-based designed inhibitors. The benefit acquires deeper insights into the structure-activity relationships.
Reference:
Chem Sci. 2019 Feb 13;10(12):3573-3585.