Myeloid cell leukemia 1 (MCL1) is a prosurvival protein. It overexpresses in a variety of different cancers and is of tremendous therapeutic interest. Furthermore, MCL1 is involved in complex protein−protein interactions (PPIs) involving proapoptotic factors Bim, Bak, and Bax. Thus, MCL1 is a vital survival factor in human cancers, such as lymphoma, leukemia, breast cancer, and multiple myeloma (MM), wherein levels of MCL1 directly correlate to disease progression. On the one hand, some compounds modulate MCL1 through competitive inhibition of interactions with its pro-apoptotic targets. It disrupts “hot spots” of the PPI interfaces. On the other hand, the recent surge in applications toward selective protein degradation, especially since seminal reports utilizing proteolysis targeting chimera (PROTAC) technology. dMCL1-2 is a potent and selective degrader of MCL1 based on PROTAC.
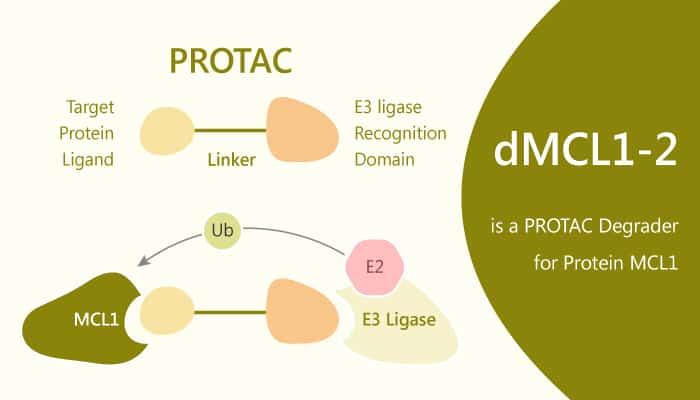
PROTACs are small molecule conjugates that tether target proteins and E3 ligases through hetero-bifunctional poles. They induce ubiquitination and label proteins for proteasomal degradation. Here, the authors demonstrate the development of PROTACs capable of inducing degradation of the antiapoptotic protein MCL1. In addition, dMCL1-2 binds to MCL1 with a KD of 30 nM. Furthermore, it forms ternary complex between CRBN and MCL1, necessary for PROTAC-mediated degradation.
dMCL1-2, a PROTAC which effectively enhances proximity between MCL1 and the E3 ligase CRBN. It induces direct ubiquitination of MCL1 and labels it for proteasomal degradation at nanomolar concentrations. Moreover, dMCL1-2 induces apoptosis at 250 and 500 nM after a 24 h treatment with 1% fetal bovine serum in OPM2WT cells. It reveals by cleavage of Caspase-3.
In summary, dMCL1-2 is the first demonstration of a PROTAC. It will be a powerful tool for studying this family of antiapoptotic proteins. Meanwhile, it provides a more indepth understanding into their mechanisms of action.
Reference:
Papatzimas JW, et al. J Med Chem. 2019 Jun 13;62(11):5522-5540.