Both CBP (Cyclic AMP response element binding protein, binding protein) and P300 (paralog E1A-associated protein of 300 kDa) possess several structured regions. As such, the histone acetyltransferase (HAT) domain is a common region. It acetylates both histone and non-histone proteins, and bromodomain (BRD) that binds acetylated lysine (KAc). In fact, CBP and P300 have been implicated as potential cancer treatment targets. A study from Kwong Wah Lai discovered and designed an inhibitor of the bromodomain of CBP GNE-207.
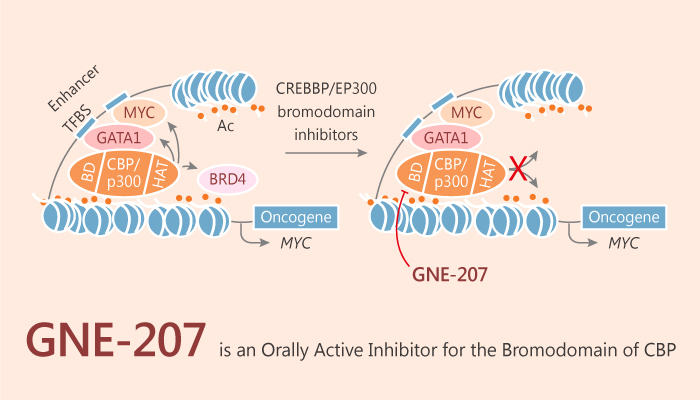
In viro, GNE-207 is a potent, selective and orally bioavailable inhibitor of the bromodomain of CBP, with an IC50 of 1 nM, a selectively index of >2500-fold against BRD4 (1) (IC50, 3.1 μM). At the same time, GNE-207 shows excellent CBP potency, with an EC50 of 18 nM for MYC expression in MV-4-11 cells.
In vivo, GNE-207 exhibited moderate clearance in PK, with acceptable oral bioavailability. In addition, GNE-207 with the dosage of 5 mg/kg shows moderate clearance in PK, with acceptable oral bioavailability. The authors also compared the biological activity GNE-207 with GNE-781. Not surprisingly, GNE-207 (compound 35) did have an improved half-life compared to GNE-781; the selectivity for CBP over BRD4 was less than the selectivity that GNE-781 exhibited. Importantly, GNE-207 became an instrumental in vitro tool representing a distinct chemical series. More strikingly, PK results in mouse of selected inhibitors revealed that combination of Isoquinoline and pyridine, as exemplified by GNE-207, can complement each other in increasing volume of distribution and half-life.