PRMT6 is a protein arginine methyltransferase responsible for histone H3 arginine 2 (H3R2) methylation. PRMT6 negatively regulates DNA methylation and that PRMT6 upregulation contributes to global DNA hypomethylation in cancer. Mechanistically, PRMT6 overexpression impairs the chromatin association of UHRF1, resulting in passive DNA demethylation. Especially, PRMT6 plays an important role in several biological processes associated with multiple cancers. In this study, researchers discovered the first potent, selective, and cell-active covalent inhibitor of PRMT6, MS117.
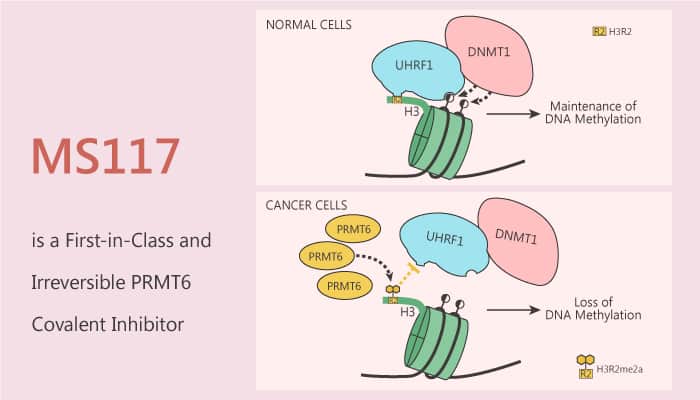
PRMT6 overexpression impairs the Uhrf1 association with chromatin. In particular, MS117 is a potent PRMT6 inhibitor and remarkably selective for PRMT6 over 28 PKMTs, DNMTs, and type II and III PRMTs. In addition, MS117 shows good selectivity for PRMT6 (IC50=0.018 μM) over PRMT3 (IC50=3 μM) and PRMT4 (IC50=0.48 μM). Moreover, MS117 shows moderate selectivity (6-fold) for PRMT6 over PRMT1 (IC50=0.1 μM) and PRMT8 (IC50=0.11 μM).
MS117 potently and concentration-dependently reduces cellular levels of H3R2 asymmetric dimethylation (H3R2me2a, IC50=1.3 μM). Furthermore, MS117 treatment results in a concentration-dependent inhibition of H4R3me2a levels with an IC50 value of 5.6 μM, indicating that the compound is less potent at inhibiting PRMT1 than PRMT6 in cells. MS117 (at concentrations up to 20 μM for 3 days) is not cytotoxic in MCF-7, PNT2, and HEK293T cells. Besides, MS117 is a valuable chemical tool without significant cell toxicity. In cellular assays, MS117 potently inhibits PRMT6, is less potent against PRMT1 and inactive against PRMT3 and PRMT4, and is not cytotoxic in general.
Collectively, MS117 is a first-in-class PRMT6 covalent inhibitor and a valuable tool compound for the biomedical community to investigate PRMT6 biological functions and disease associations.