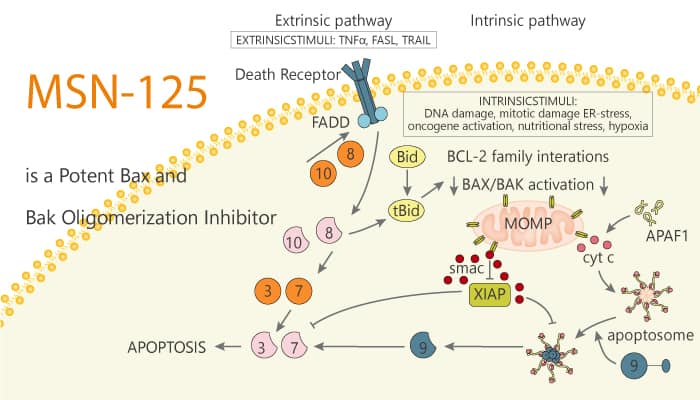
Aberrant apoptosis can lead to acute or chronic degenerative diseases. Apoptosis largely divides into two pathways ultimately leading to caspase activation and subsequent cellular disintegration. Extracellular signals activating death receptors triggers the extrinsic pathway, and intracellular stress activates the intrinsic pathway. It largely regulated at the mitochondrial outer membrane (MOM) by the pro- and anti-apoptotic members of the B cell lymphoma 2 (Bcl-2) family of proteins. The MOM permeabilization (MOMP) is the first irreversible step in apoptosis. MOMP results from an ordered series of steps beginning with the activation of one or more Bcl-2 homology 3 proteins (BH3-proteins) or releasing previously activated pro-apoptotic proteins Bax or Bak from inhibition by an anti-apoptotic protein of the Bcl-2 family. Therefore, directly inhibiting Bax and Bak would be a more efficient approach to inhibit MOMP. In this study, MSN-125 is a potent Bax and Bak oligomerization inhibitor.
MSN-125 prevents mitochondrial outer membrane permeabilization.
MSN-125 efficiently inhibits liposome permeabilization. In addition, it prevents MOMP with an IC50 of 4 μM. MSN-125 potently inhibits Bax/Bak-mediated apoptosis in HCT-116, BMK Cells, and primary cortical neurons protect primary neurons against glutamate excitotoxicity. In reactions containing liposomes and tBid, the addition of MSN-125 reduces FRET between DAC-Bax-134C and NBD-Bax-126C. MSN-125 disrupts helix 6 and helix 9 interactions, and some, but not all, interactions in the BH3-groove interface. Moreover, MSN-125 can serve as an important tool for identifying molecular interactions critical for forming functional Bax oligomers.
In summary, MSN-125 is a potent Bax and Bak oligomerization inhibitor. MSN-125 interferes with correct dimer formation for Bax and Bak, thereby inhibiting further oligomerization and preventing MOMP. Therefore, it provides novel tools for the investigation of the mechanisms leading to MOMP and will ultimately facilitate the development of compounds inhibiting Bax/Bak in acute and chronic degenerative diseases.
Reference:
Niu X, et al. Cell Chem Biol. 2017 Apr 20;24(4):493-506.e5.