Cysteine proteases implicate in apoptosis and cytokine processing. Caspase-8 is a member of the cysteine proteases. In particular, Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Z-LE(OMe)TD(OMe)-FMK is a selective caspase-8 inhibitor. Besides, Z-LE(OMe)TD(OMe)-FMK shows its antiproliferative and cytotoxic activity via induction of apoptosis.
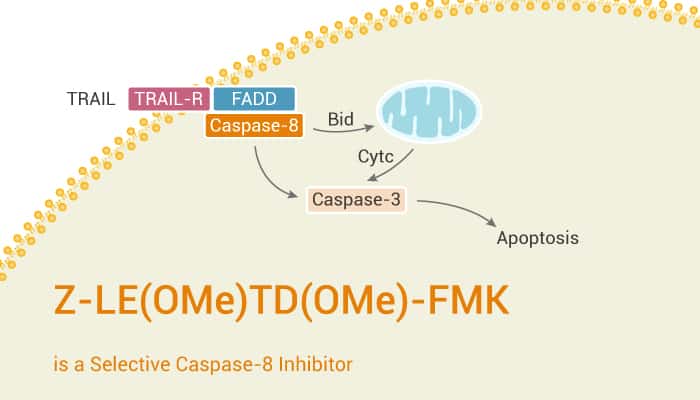
Caspase-8 is the initiator caspase of extrinsic apoptosis. Caspase-8 inhibits necroptosis mediated by RIPK3 and MLKL. Like all caspases, caspase-8 is synthesized as an inactive single polypeptide chain zymogen procaspase. Furthermore, Procaspase-8 binds to the exposed death effector domain (DED) of death receptor-associated Fas-associated via death domain (FADD). This binding acts through a pocket in its DED1, forming the death-inducing signaling complex (DISC). Then, additional procaspase-8 molecules recruit and bind through a second DED1 pocket to a motif in DED2 of the prior procaspase-8 by dominant hydrophobic interactions. Moreover, this results in DED-mediated procaspase-8 oligomerization. The binding of procaspase-8 to FADD thus prompts the recruitment of additional procaspase-8 molecules that leads to the formation of a filament assembled in a unidirectional manner.
Moreover, caspase-8-/- mice die in utero as a result of defective development of heart muscle and display fewer hematopoietic progenitor cells. In addition, Inhibition of the caspase-8 activity protects against Fas receptor–mediated apoptosis in vivo.
Taken together, the anti-apoptotic functions of caspase-8 may act as a critical block to existing antitumor therapies. Importantly, reversal or inhibition of caspase-8 phosphorylation may prove a valuable avenue to explore for sensitization of resistant tumors to extrinsic apoptotic stimuli.