Aberrations in post-translational modifications by ubiquitin or ubiquitin-like proteins (Ubl), such as the small ubiquitin-like modifiers (SUMO) relate to the pathogenesis of lifethreatening diseases, such as cancer, neurodegenerative disorders, and viral infection. For instance, emerging studies have identified the dyregulation of SUMOylation in various cancers. Additionally, the SUMO-activating enzyme (SAE, SUMO E1) is hopeful to be a potential target to inhibit c-Myc- and KRas-dependent cancers. Surprisingly, it also could reduce cancer cell stemness and resistance. Despite the importance of Ubl modifications in dysregulated signaling pathways been identified, only a few U.S. Food and Drug Administration-approved drugs on this target have been developed. In other words, to find novel related active compounds targeted ubiquitin or ubiquitin-like proteins is urgent and essential.
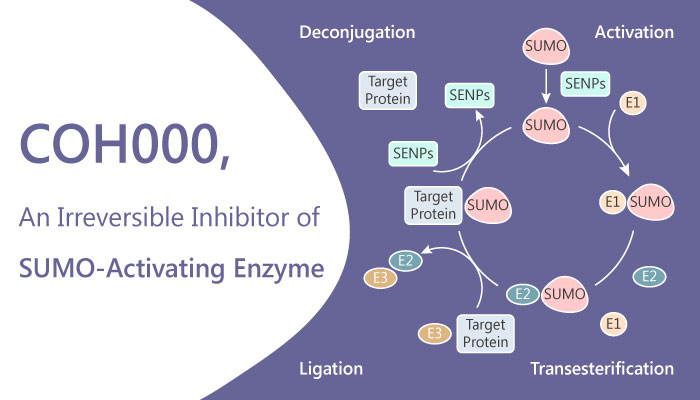
A study from Yi-Jia Li, et al. who come from the Beckman Research Institute, have identified a allosteric inhibitor of ubiquitin-like 1-activating enzyme (SUMO-activating enzyme), COH000. COH000 is an allosteric, covalent and irreversible inhibitor of Ubiquitin-like 1-activating enzyme (SUMO-activating enzyme), with an IC50 of 0.2 μM for SUMOylation in vitro. In addition, COH000 has over 500-fold selectivity for SUMOylation than ubiquitylation. Furthermore, COH000 inhibits SUMO adenylation without directly competing with ATP or SUMO1 binding. Not only that, the allosteric and non-ATP competitive inhibitor also shows excellent anti-tumor activity in xenograft colorectal cancer models in mice. In addition, in miRNA regulation levels, COH000 induces miR-34b expression and reduces c-Myc expression. And it demonstrates anti-proliferation activity in cells.
To conclude, COH000 is a promising preclinical candidate in the treatment of colon cancers. However, there are still many problems to solve and improve. We are looking forward to more research efforts to be attempted.