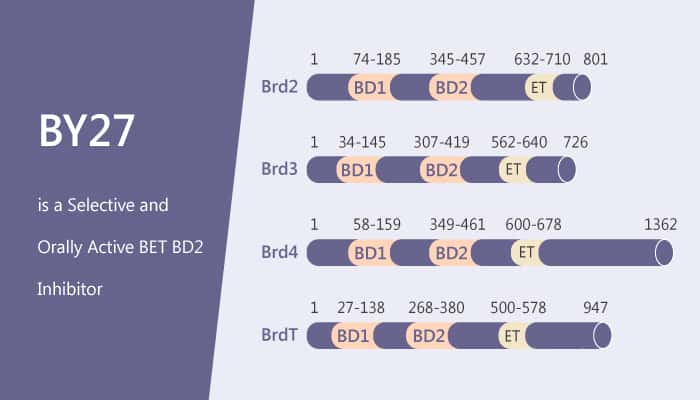
BET (Bromodomain and extra terminal domain) is a well-known member of the bromodomain family. It contains BRD2, BRD3, BRD4, and BRDT, which have two tandem bromodomains (BD1 and BD2). BD1 and BD2 are recognition motifs for the acetyl-lysine residues of N terminuses of histones as well as other proteins. The BD1 domains of BET share a similar amino acid sequence, while it is the same trend with BET BD2 domains. BET proteins play important role in biological functions. Once dysfunction happens, the proteins cause various diseases, especially cancers.
In the past decades, scientists reported numerous BET inhibitors. Many of them have already in clinical trials. These demonstrate that small compounds have a potential role in the treatment of human diseases. However, most of the BET inhibitors have similar affinities at both BET BD1 and BET BD2. They are pan-inhibitors and have no advantages over the selective compounds. Therefore, it is a need to explore more selective inhibitors for further study.
Recently, Deheng Chen et al synthesized a series of analogs based on the structures of BET inhibitors already known. Then, they examined the affinity to BD1 and BD2 of BET. Subsequently, they discovered BY27 as a selective BET BD2 inhibitor. Herein, we are going to talk about the promising compound.
BY27 is a potent, selective BET BD2 inhibitor, shows 38, 5, 7, and 21-fold BD1/BD2 selectivity for BRD2, BRD3, BRD4, and BRDT. It shows no effect on non-BET proteins.
Meanwhile, BY27 exhibits excellent oral bioavailability and anti-tumor activity in the animal assay. It inhibits the growth of the tumor with less toxicity.
References:
1. Chen D, et al. Eur J Med Chem. 2019 Aug 21;182:111633.