ETS transcription factors mediate a wide array of cellular functions and are attractive targets for pharmacological control of gene regulation. Transcription factors (TFs) are central to many cellular processes and account for 5-10% of genes in eukaryotes. Besides, sequence-specific binding is an obligate step in ETS-mediated gene activation. So inhibiting the appropriate ETS–DNA complex with small molecules offers significant potential to impact therapy for a broad range of diseases. Moreover, all ETS proteins share a conserved DNA binding domain that recognizes sites harboring a 5′-GGAA/T-3′ consensus. Like all ETS proteins, PU.1 binds sequence specifically to 10-bp sites by inserting a recognition helix into the major groove of a 5′-GGAA-3′ consensus. Furthermore, It accompanied by contacts with the flanking minor groove. DB1976 is a selenophene analog of DB270 and a potent and cell-permeable fully efficacious transcription factor PU.1 inhibitor.
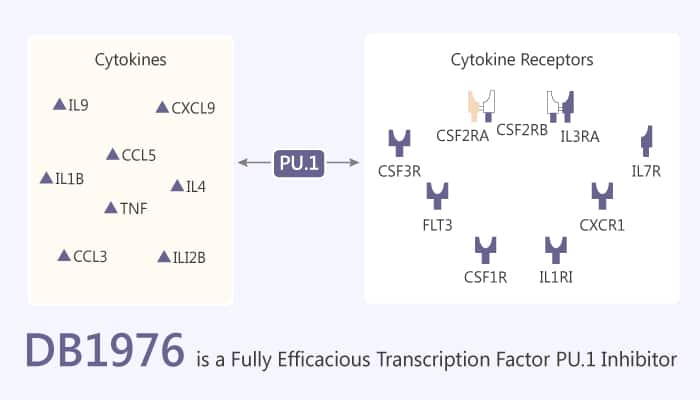
DB1976 is a selenophene analog of DB270 and a potent and cell-permeable fully efficacious transcription factor PU.1 inhibitor. In addition, DB1976 potently inhibits PU.1 binding (IC50 of 10 nM) and strongly inhibits the PU.1/DNA complex (with high DB1976-λB affinity, KD of 12 nM) in vitro. Meanwhile, DB1976 has an apoptosis-inducing effect. Similarly, the occupancy of DB1976 at sites flanking both sides of the ETS consensus induces conformational changes in the intervening sequence. In summary, DB1976 inhibits PU.1-specific gene transactivation in live cells, and do so without toxicity. Nonetheless, DB1976 is largely localized similarly to DOX in the nucleus. All in all, DB1976 is a potent and cell-permeable fully efficacious transcription factor PU.1 inhibitor.
References:
Munde M, et al. Nucleic Acids Res. 2014 Jan;42(2):1379-90.