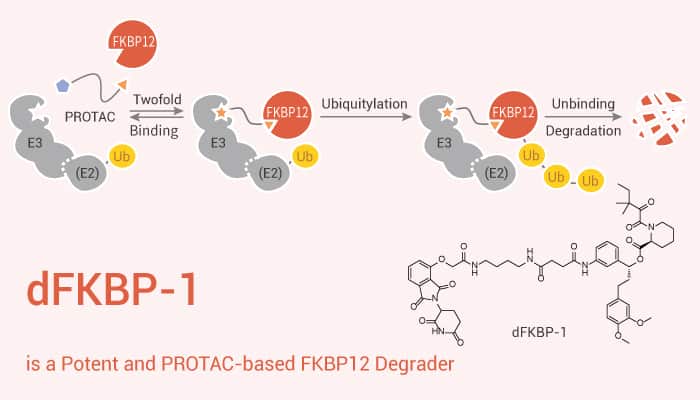
The FK506-binding protein 12 (FKBP12) is a ubiquitous abundant protein that acts as a receptor for the immunosuppressant drug FK506l FK506 binds tightly to intracellular calcium release channels and to the TGF-β type I receptor. dFKBP-1 is a potent and PROTAC-based FKBP12 degrader. FKBP12 inhibits the basal signaling of these three receptors and also regulates the P-glycoprotein multidrug transporter. Besides, FKBP12 occurs in very high concentrations in all cells and so might be expected to regulate fundamental aspects of cell biology.
FKBP12 is a cytoplasmic protein and possesses multiple functions in signaling transduction based on its interaction with different cellular targets. Moreover, FKBP12 interacts with oncoprotein MDM2 and induces MDM2 degradation. FKBP12 degrades MDM2 through binding to MDM2 protein, disrupting MDM2/MDM4 interaction and inducing MDM2 self-ubiquitination. In addition, FKBP12 also significantly inhibits endogenous MDM2. Furthermore, FKBP12 also inhibits the enhanced level of MDM2 following p53 activation in nutlin-3 treated cells.
In this study, researchers identify a novel function for FKBP12 in downregulating MDM2. dFKBP-1 directly enhances sensitivity of cancer cells to chemotherapy and nutlin-3 treatment. In particular, dFKBP-1 potently decreases FKBP12 abundance in MV4;11 cells, leading to over 80% reduction of FKBP12 at 0.1 μM and 50% reduction at 0.01 μM. Pre-treatment with Carfilzomib, MLN4924, free SLF or free Thalidomide rescues the destabilization of FKBP12 by dFKBP-1. Treatment of 293FT-WT cells with dFKBP-1 induces potent, dose-dependent degradation of FKBP12.
To summarise, FKBP12 binds to most of the rapalogs with high affinity, creating a large repertoire of composite surfaces for potential recognition of macromolecular targets such as proteins.