Mitochondria provide cellular energy through its oxidative phosphorylation system, which needs to coordinate the expression of genes encoded by nuclear and mitochondrial genomes (mtDNA). Specifically, the transcription of mtDNA in circular mammals depends on a single mitochondrial RNA polymerase (POLRMT). Besides, three proteins are required for mitochondrial transcription initiation: POLRMT, TFB2M, and TFAM.
Structurally, POLRMT consists of four main domains: N-terminal extension, pentatricopeptide repeat (PPR) domain, N-terminal domain and C-terminal domain. Moreover, the interaction between POLRMT and TFAM is mediated by the N-terminal extension domain of POLRMT. After the binding of TFB2M with dsDNA, TFB2M induced the structural changes of stable open DNA region in POLRMT. Furthermore, the interaction between TFAM and POLRMT prevents the production of replication primers, so it is suggested to be a molecular switch between replication and transcription. In vitro studies have shown that POLRMT plays an important role in mitochondrial DNA replication. Here, we will introduce a first-in-class specific and noncompetitive human POLRMT inhibitor, IMT1.
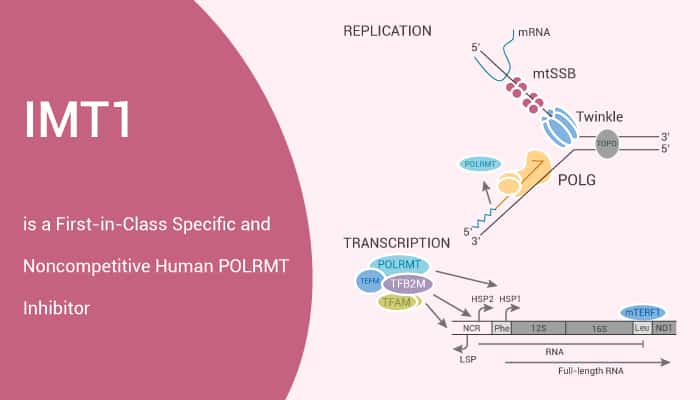
IMT1 is a First-in-Class Specific and Noncompetitive Human POLRMT Inhibitor.
At first, IMT1 causes a conformational change of POLRMT, which blocks substrate binding and transcription in a dose-dependent way in vitro. Meanwhile, IMT1 reduces deoxynucleoside triphosphate levels and citric acid cycle intermediates. This results in a marked depletion of cellular amino acid levels. Nonetheless, IMT1 has the potential for mitochondrial transcription disorders-related diseases.
Secondly, IMT1 with 0.00001-10 μM for 0-168 h has a dose-dependent decrease in cell viability in A2780, A549, and HeLa cells. Interestingly, IMT1 shows a strong decrease in cell viability in about one-third of the cancer cell lines, 89 cancer cell lines, and primary cells. Importantly, primary cells remained unresponsive. Particularly, IMT1 causes a dose-dependent decrease in the levels of mitochondrial transcripts and gradual depletion of mtDNA in HeLa cells. There is a dose-dependent decrease in the levels of subunits (NDUFB8, UQCRC2, and COXI) of respiratory chain complexes I, III, and IV.
Thirdly, IMT1 reveals a time-dependent and marked increase in the levels of mono- and diphosphate nucleotides. Obviously, this results in a considerable increase in the AMP/ATP ratio and levels of phosphorylated AMPK in A2780 cells. IMT1 severely impairs mtDNA gene expression in A2780 cells that express wild-type POLRMT. Additionally, cells that express mutant POLRMT (L796Q or L816Q) are resistant. POLRMT is essential for mtDNA transcription and biogenesis of the oxidative phosphorylation (OXPHOS) system.
IMT1 is a first-in-class specific and noncompetitive human POLRMT inhibitor.
References:
Nina A Bonekamp, et al. Nature. 2020 Dec;588(7839):712-716.