Melanomas were responsible for more than 75% of estimated skin cancer deaths in 2012. And the incidence rate has been increasing for the last 30 years. Additionally, NRAS is the second most frequently mutated gene in melanoma. Researcheres also have demonstrated the sensitivity of cancer cell lines carrying KRAS mutations to apoptosis initiated by inhibition of protein kinase C delta (PKCδ).A study from Asami Takashima discovered and identified a potent and selective PKCδ inhibitor, BJE6-106 (B106).
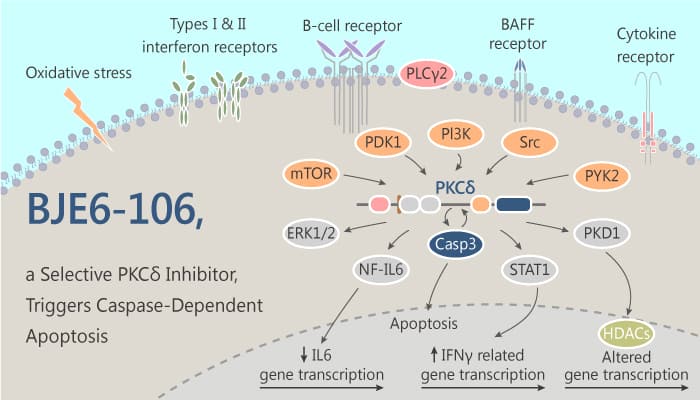
In the study, the authors demonstrated that PKCδ inhibition is cytotoxic in melanomas with primary NRAS mutations. For instance, inhibition of PKCδ suppressed the growth of multiple melanoma cell lines carrying NRAS mutations, mediated via caspase-dependent apoptosis.
In vitro, BJE6-106 (B106) is a potent, selective 3rd generation PKCδ inhibitor with an IC50 of 0.05 μM and targets selectivity over classical PKC isozyme PKCα. BJE6-106 (B106) (0.2 μM, 0.5 μM; 24-72 hours) suppresses cell survival in melanoma cell lines with NRAS mutations. In addition, BJE6-106 (B106) (0.2 μM, 0.5 μM; 6-24 hours) triggers caspase-dependent apoptosis, increases the activity of caspase 3/7, the effect of B106 is greater than rottlerin (10-fold) in SBcl2 cells. Moreover, BJE6-106 (B106) (0.5 μM; 2-10 hours) activates the MKK4-JNK-H2AX Pathway by inducing MKK4, JNK and H2AX activation at different times in SBcl2 cells.
Taken together, PKCδ is a therapeutic target in melanomas with NRAS mutations or BRAF inhibitor resistance. Among the novel PKCδ inhibitors, BJE6-106 exhibited excellent anti-tumor activity. More in vivo experiments need to be verified.