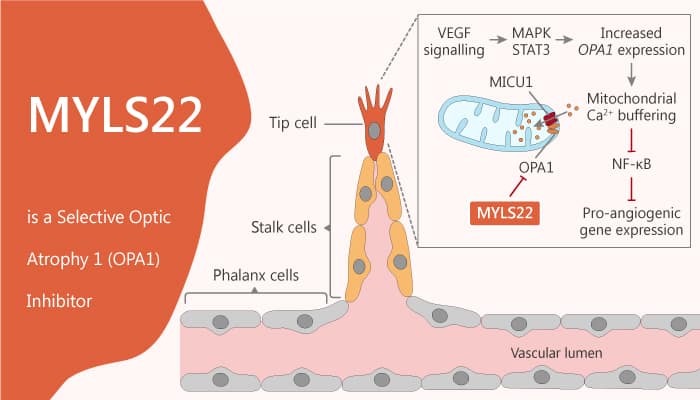
Optic neuropathy is damage to the optic nerve from any cause. Specifically, damage and death of these nerve cells, or neurons, leads to characteristic features of optic neuropathy. Angiogenesis requires an internal mitochondrial membrane mitochondrial fusion protein optic atrophy 1 (OPA1). Besides, mitochondrion fusion is controlled by mitochondrion fusion element (MFN) 1 and 2 in OMM and passes through the optic OPA1 membrane (IMM) in mitochondria. In response to angiogenic stimulation, the OPA1 level increased rapidly. Moreover, it restricted the nuclear factor kappa light chain enhancer of activated B cells (NF-κb) signal transduction. Furthermore, it finally resulted in the expression of angiogenic genes and angiogenesis. Indeed, endothelial OPA1 required in NF-κ B-dependent pathways that affect development and tumor angiogenesis, tumor growth, and metastasis. MYLS22 is a first-in-class and selective optic atrophy 1 (OPA1) inhibitor.
MYLS22 is a first-in-class and selective optic atrophy 1 (OPA1) inhibitor with anti-angiogenesis and anti-cancer activity.
How does MYLS22 work on the target? Let’s study it together. In the beginning, MYLS22 is a first-in-class and selective OPA1 inhibitor. Meanwhile, MYLS22 inhibits angiogenesis and tumor growth and metastatization, by impinging on NFkB activity and on angiogenic gene expression. In addition, MYLS22 causes tumor growth curtailed mice with 10 mg/kg/die. Nonetheless, MYLS22 did not display any additive effect in Opa1+/DEC mice, further confirming its specific Opa1 targeting. Particularly, The effect of myls22 on the growth of implanted melanoma was completely opposite to that of OPA1. All in all, MYLS22 is a first-in-class and selective optic atrophy 1 (OPA1) inhibitor with anti-angiogenesis and anti-cancer activity.
References:
Stéphanie Herkenne, et al. Cell Metab. 2020 May 5;31(5):987-1003.e8.