Immunomodulatory drugs (IMiDs), including Thalidomide and its analogues, which possess pleiotropic anti-myeloma properties. These properties include immune-modulation, anti-angiogenic, anti-inflammatory and anti-proliferative effects. Thalidomide is an immunomodulatory drug, which is used mainly in the treatment of multiple myeloma. The proliferation of clonal plasma cells causes multiple myeloma. Multiple myeloma leads to anemia, renal failure, hypercalcemia and destructive bone lesions resulting in significant morbidity. Immunomodulatory drugs exert anti-myeloma activity by binding to the protein cereblon (CRBN) and subsequently degrading IKZF1/3. The unique ability of immunomodulatory drugs to bind to CRBN leads to the development of the proteolysis targeting chimeras (PROTACs) technology. PROTAC is a bi-functional molecule with two ligands–one for the target protein, and the other for E3 ubiquitin ligases. PROTACs induce proteasomal degradation of the target protein.
Recently, many researchers study the degradation of BET proteins in multiple cancer cells by highly potent PROTACs with Thalidomide analogues. In particular, the deregulation of BRD4 protein activity in cancer and inflammatory diseases makes BET proteins attractive therapeutic targets. Moreover, BRD4 is the most commonly-targeted protein in PROTAC technology. BRD4 is a member of the bromodomain and extra-terminal domain (BET) family.
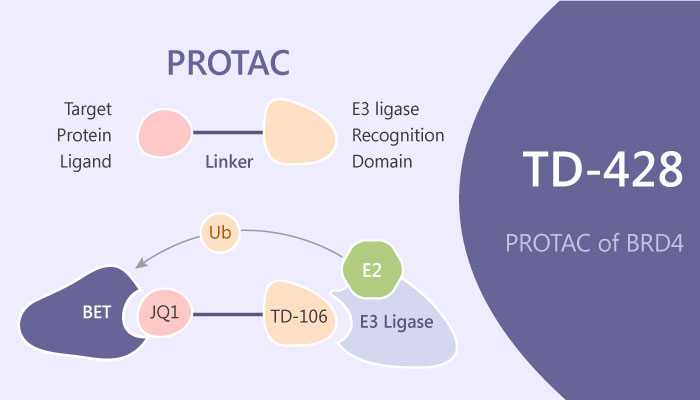
In this study, Sung AhKim, et al designd and synthesized a novel immunomodulatory drug analog TD-106. Firstly, TD-106 induces the degradation of IKZF1/3 and inhibits the proliferation of multiple myeloma cells in vitro as well as in vivo. Moreover, Sung AhKim, et al demonstrated that TD-428, which comprises TD-106 linked to a BET inhibitor, JQ1 efficiently induce BET protein degradation in the prostate cancer cell line. Gratifyingly, TD-428 reduces c-Myc levels more efficiently than JQ1. Besides, TD-428 induces more potent degradation of BRD4 with a DC50 of 0.32 nM.
Reference:
Sung AhKim, et al. Eur J Med Chem. 2019 Mar 15;166:65-74.