PRMT5 is the main type II enzyme. It catalyzes symmetric dimethylarginine of histone proteins to induce gene silencing by generating repressive histone marks. PRMT5 can also methylate nonhistone proteins such as the transcription factors p53 and p65. Modifications of these proteins by PRMT5 have relations with diverse cellular processes. Targeting PRMT5 by specific inhibitors has emerged as a potential therapeutic strategy to treat these diseases. In addition, The MTAP gene is proximal to and co-deleted in nearly all CDKN2A-deleted cancers. This genetic alteration is present in an estimated 9% of all cancers. MTAP is required for the methionine salvage pathway and MTAP-del cells accumulate MTA, an inhibitory co-factor which competes for binding to the co-factor binding site of PRMT5 with the activating co-factor SAM. PRMT5 also adds symmetric dimethylarginine (SDMA) modification to proteins and is essential for mammalian cell survival. MRTX9768 is a potent, orally active PRMT5 inhibitor.
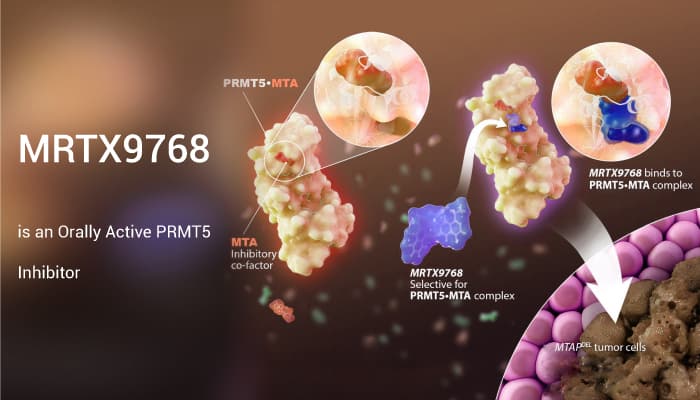
MRTX9768 is a synthetic lethal-based inhibitor. It binds the PRMT5-MTA complex and also selectively targets MTAP/CDKN2A-deleted tumors. In addition, it is a potent inhibitor of SDMA and cell proliferation in HCT116 MTAP-del cells (SDMA=IC50: 3 nM; prolif=IC50:11 nM) with selectivity over HCT116 MTAP-WT cells. Furthermore, in xenograft studies, oral administration of MRTX9768 demonstrates dose-dependent inhibition of SDMA in MTAP-del tumors, with less SDMA modulation observed in the bone marrow.
In summary, multiple independent research teams have demonstrated that tumor cell lines with homozygous MTAP deletions are hypersensitive to shRNA-mediated knockdown of PRMT5. MRTX9768 is an orally active PRMT5•MTA inhibitor, and it has selective antitumor activity in MTAP-del tumor cells while sparing MTAP-WT cells.
Reference: