Androgen receptor (AR) signaling is crucial for normal prostate development. It also drives the growth and survival of prostate cancer cells. AR signaling suppression is a common strategy for treating prostate cancer. Like other occupancy-based inhibitors, the antiandrogen enzalutamide requires high saturating drug concentrations to achieve its clinical benefit. In addition, AR remains the principal driver of metastatic disease. Unfortunately, this need to achieve and maintain high systemic concentrations is one of the major challenges in drug development today. However, in recent years, there emerges an alternative potential therapeutic approach, i.e., induced protein degradation. Proteolysis Targeting Chimeras (PROTACs) are heterobifunctional molecules that work by creating a trimeric complex between a target protein and an E3 ubiquitin ligase, thus facilitating target ubiquitination and subsequent degradation. PROTAC engagement leads to target protein degradation. In this study, ARCC-4 is an AR degrader based on PROTAC, with a DC50 of 5 nM.
ARCC-4 is a low-nanomolar AR degrader, and effectively degrades clinically relevant AR mutants associated with antiandrogen therapy.
ARCC-4 is an Enzalutamide-based von Hippel-Lindau (VHL)-recruiting AR PROTAC and outperforms Enzalutamide. It is able to target clinically relevant mutants of AR, and that protein degradation could have therapeutic benefits in mutational contexts that are not susceptible to AR inhibition. ARCC-4 induces apoptosis and inhibiting the proliferation of AR-amplified prostate cancer cells. It enhances protein-protein interactions between AR and VHL, thereby promoting the association of the trimeric complex. In addition, it potently degrades AR with a D50 of 5 nM and Dmax of over 95%. Meanwhile, ARCC-4 shows near-complete AR degradation (>98%) in prostate cancer cells. it selectively degrades AR via the proteasome but not PR-A or PR-B suppression. ARCC-4 shows efficacy against clinically relevant AR mutations.it also maintains activity despite elevated androgen levels.
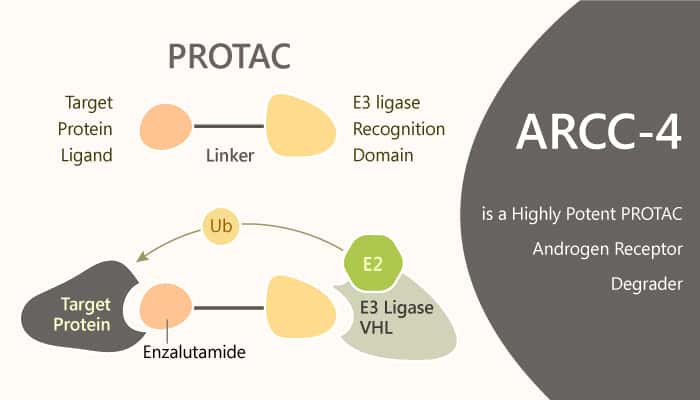
In summary, ARCC-4 binds to the AR ligand-binding domain in competition with high-affinity androgens. ARCC-4 will be useful as a tool compound to probe AR biology. It is also useful for dissecting the in vitro cellular mechanisms of diseases that rely on AR, such as prostate cancer, breast cancer, and spinal bulbar muscular atrophy. Thus, the PROTAC approach is better able to ablate AR signaling in the presence of androgens.
Reference:
Salami J, et al. Commun Biol. 2018 Aug 2;1:100.