Come straight to the point, CC-90010 (compound 1) is a reversible and orally active BET inhibitor. CC-90010 is applied in the study for advanced solid tumors.
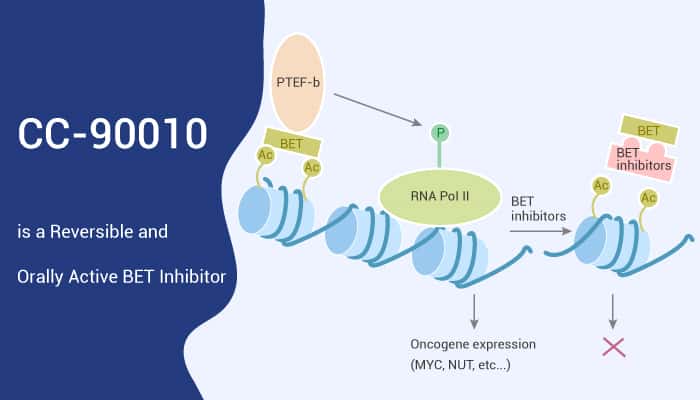
The bromodomain and extra-terminal domain (BET) proteins, epigenetic readers, recognize and bind acetylated lysine residues. The BET protein family comprises the ubiquitously expressed BRD proteins BRD2, BRD3, and BRD4, as well as testis-restricted BRDT. BET proteins also play a vital role in cancer, involved in regulating proliferation, metabolism, cancer stem cells, and metastasis. Especially, the most famous member, BRD4, plays a key role in the super-enhancer organization and in regulating c-myc. C-myc is an essential oncogene in many tumor types.
Plenty of preclinical studies suggesting the role of BET proteins in cancer pave a way for developing and using BET inhibitors as antitumor drugs. BET inhibitors specifically bind bromodomains, preventing BET proteins from binding to chromatin, thereby inhibiting gene transcription. BET inhibitors have broad anticancer activity in preclinical studies; however, first-generation inhibitors have generally shown modest clinical benefit.
CC-90010 has significant antiproliferative activity in glioblastoma cells and PDX models as monotherapy and in combination with temozolomide. In addition, CC-90010 seems to penetrate the BBB Structurally differentiated next-generation BET inhibitors, such as CC-90010, have the potential to achieve robust target engagement and thus significant antitumor activity by leveraging different dosing schedules. Moreover, the first-in-human phase I study of CC-90010 is under conducted in patients with advanced or unresectable solid tumors or relapsed/refractory (R/R) advanced non-Hodgkin’s lymphoma (NHL).
Reference:
- CELGENE QUANTICEL RESEARCH, INC. PROCESS FOR THE PREPARATION OF BROMODOMAIN INHIBITOR. Patent. WO2020023438.
- Moreno, et al. CC-90010, a reversible, oral bromodomain and extra-terminal (BET) inhibitor in patients (Pts) with advanced solid tumours (STs) and relapsed/refractory (R/R) non-Hodgkin lymphoma: Updated results of a phase I study.