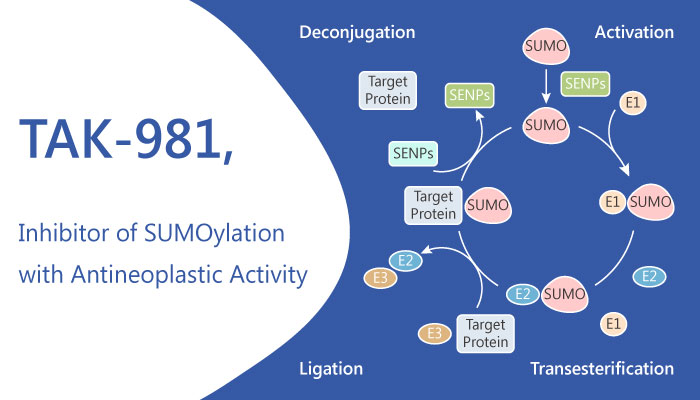
Recently, National Cancer Institute has reported a novel inhibitor TAK-981 for SUMOylation enzymatic cascade. This inhibitor showed potential immune-activating and antineoplastic activities.
Firstly, TAK-981 targets and covalently binds to the small ubiquitin-like modifier (SUMO; small ubiquitin-related modifier) protein, forming an adduct with SUMO. This binding blocks the transfer of SUMO from the SUMO-activating enzyme (SAE) to SUMO-conjugating enzyme UBC9. This inhibition abrogates many sumoylated protein-mediated cellular processes that participate in tumorigenesis, including cell proliferation, metastasis and survival. In addition, TAK-981 could increase type 1 IFN-mediated signaling by upregulating the production of type 1 interferon (IFN). Therefore, the inhibitor activates innate effector cells and enhances the antitumor innate immune responses.
Additionally, in both mouse bone-marrow and human peripheral blood mononuclear cell derived dendritic cells (DCs), TAK-981 induced the activation and maturation of CD40, CD80 and CD86. Furthermore, this compound upregulated inflammatory cytokines including IP-10, MCP1, MIP-α, MIP-β, IFNα and IFNβ. IFNAR blocking antibody could reverse the effects, demonstrating dependence of TAK-981 induced pharmacodynamics effects on Type I interferon signaling in DCs. In vivo, a single administration of TAK-981 hypodermically in naïve Balb/c mice at the brachial lymph nodes induced activation of DCs, in CD40 and CD86 expression. Concomitantly, an significant increase of CD69 was observed on T cells and treated with TAK-981 ex vivo.
These results suggest that TAK-981 may promote anti-tumor immune responses in mice via enhanced cross-presentation of exogenous antigens released by dying tumor cells. Therefore, the effect leads to priming and activation of antigen reactive cytotoxic T cells. At now, TAK-981 has entered in Phase 1 clinical trials in adult patients with metastatic solid tumors or lymphomas.
Reference: