HDAC (Histone deacetylases) are a group of proteins that act on histones. They remove acetyl groups from N-acetyl lysine amino acid on a histone. As a result, histones wrap DNA more tightly. The enzymes have opposite function to histone acetyltransferase. But, they both regulate DNA expression. HDAC is a large family, including four classes. HDAC 1, 2, 3 and 8 belong to Class I. Class IIa has HDAC 4, 5, 7, 9. Class IIb contains HDAC 6 and 10, and HDAC11 belong to class IV.
HDAC8 exhibits high levels in neuroblastoma patients. HDACs 6 and 10 have highly-conserved catalytic domain, play a vital role in protein degradation. HDAC10 supports neuroblastoma cell survival and promotes DNA damage repair. Thus, it is necessary to find out inhibitors of these enzymes to deal with neuroblastoma.
TH34 is a potent inhibitor of HDAC6/8/10, with IC50s of 4.6 µM, 1.9 µM and 7.7 µM, respectively. It exhibits anti-neuroblastoma activity. The compound shows much more selectivity at HDAC6/8/10 over HDAC1/2/3.
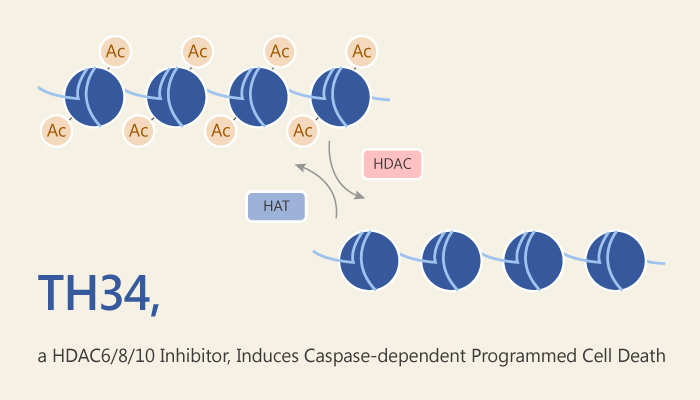
In cellular assay, TH34 causes caspase dependent programmed cell death in neuroblastoma cells. In contrast, TH34 shows no obvious cytotoxicity to normal cells. Furthermore, TH34 results in differentiation of neuroblastoma cells, and induces cell cycle arrest and mitotic aberrations in these cells. In addition, TH34 results in DNA damage in high grade neuroblastoma cells.
Summarily, TH34 is a selective HDAC6/8/10 inhibitor, has promising efficacy in the neuroblastoma study. Further research need to be done to explore more potential of the compound.
References:
1. Kolbinger FR, et al. Arch Toxicol. 2018 Aug;92(8):2649-2664.