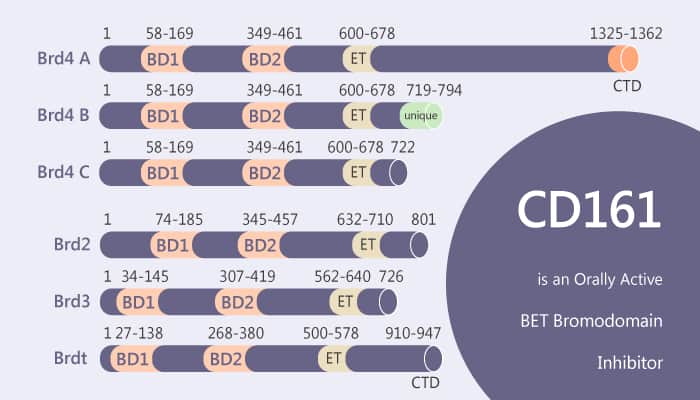
Bromodomain and extra-terminal (BET) family proteins include BRD2, BRD3, BRD4, and a testis-specific protein BRDT. In this study, researchers identify CD161. CD161 is a potent, orally bioavailable, and selective BET bromodomain inhibitor. Especially, CD161 binds to BET proteins with low nanomolar affinities and demonstrates high selectivity over 24 non-BET proteins containing bromodomains. The N-terminal domain of the BET family proteins contains two tandem and characteristic bromodomains (BRD), BD1 and BD2. Crucially, they share high sequence homology and structural similarities and are a common feature of BET proteins.
The BET BRD domains function as recognition motifs for interaction with acetylated lysine residues in histone tails and anchor their associated proteins to the target gene promoter and enhancer sites in chromatins. In particular, BET proteins are thus critical epigenetic “readers” and play a key role in the regulation of gene transcription. As a result, they are attractive new therapeutic targets for cancers and a number of other human diseases.
In recent years, researchers have developed a number of classes of potent and specific small-molecule inhibitors of BET proteins. For instance, CD161 shows potent cell growth inhibitory activity in acute leukemia cell lines harboring mixed-lineage leukemia 1 (MLL1) fusion protein and in a panel of human breast cancer cell lines. Moreover, CD161 has a good pharmacokinetic profile in mice and rats. Furthermore, CD161 demonstrates strong antitumor activity in MV4;11 acute leukemia and MDA-MB-231 breast cancer xenograft models. All in all, CD161 is a promising lead compound for further optimization.