Breast cancer is the second leading cause of cancer mortality among women worldwide. Estrogen receptor alpha (ERα) is a key hormone-regulated transcription factor for normal and malignant breast cells. Moreover, tumors that are ER-positive are much more likely to respond to treatments that block estrogen in ER-positive/HER2 negative breast cancer.
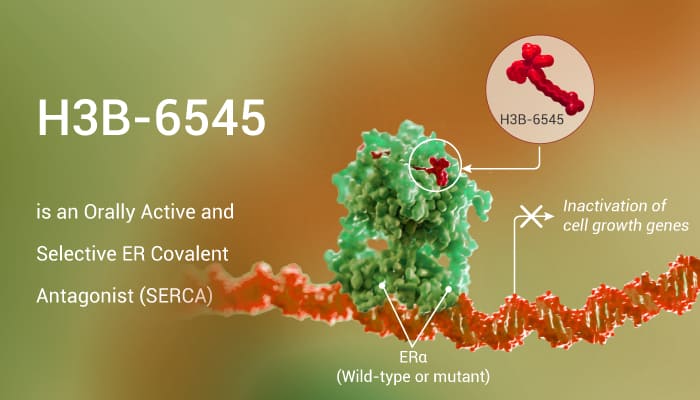
Targeted covalent inhibitors are attractive for potential enhanced biochemical efficacy, duration of action, and selectivity. Thus, All attributes to associate with their irreversible inhibition mechanism. In this study, researchers reported the discovery of H3B-6545. Especially, H3B-6545 is an orally active and selective ER covalent antagonist (SERCA). In particular, H3B-6545 inactivates both wild-type and mutant ERα for the treatment of metastatic breast cancer. In addition, H3B-6545 has a terminal elimination half-life of 2.4 h in rats and 4.0 h in monkeys. Moreover, H3B-6545 also shows low to moderate bioavailability, in line with the in vitro permeability assessment.
For in vitro metabolism, H3B-6545 (50 μM) incubates with the hepatocytes of rats and humans for 2 hours. Incubation of H3B-6545 with rat hepatocytes results in a total of 11
metabolites, including a GSH adduct. In vivo, H3B-6545 demonstrates significant single-agent anti-tumor activity in xenograft mouse models representing ERαWT and ERαY537S breast cancer. Furthermore, H3B-6545 displays dose-independent pharmacokinetics over the dose range of 1–10 mg/kg. As a result, H3B-6545 results in significant antitumor activity in both ER-positive wild-type and mutant breast cancer xenograft mouse models.
To summarise, H3B-6545 is a selective ERα covalent antagonist (SERCA) with the potential effect on bone turnover in the vivo rat model.