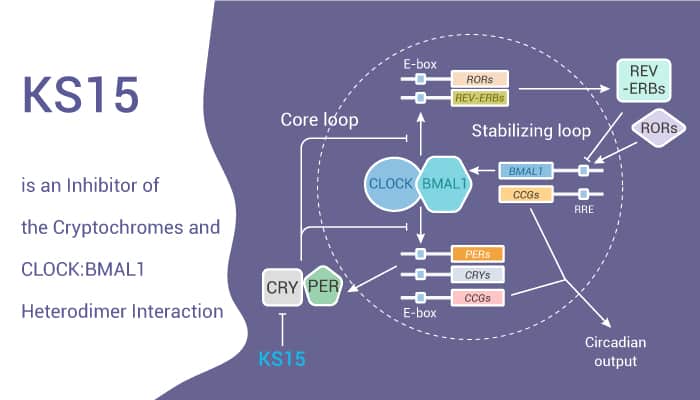
Cryptochrome (CRY), a circadian transcription factor, is a risk factor for the initiation of breast cancer. Cryptochrome inhibitor (KS15) has anti-tumor activity to human breast cancer cells.
In this study, researchers evaluated the anti-proliferative and pro-apoptotic activity of KS15 in human breast cancer cells. KS15 decreases the speed of cell growth and increases the chemosensitivity of MCF-7 cells to Doxorubicin and Tamoxifen, but has no effect on MCF-10A cells. The pharmacological inhibition of CRY by KS15 exerts an anti-proliferative effect and increases sensitivity to anti-tumor drugs in a specific type of breast cancer. KS15, an inhibitor of cryptochrome (CRY), can activate CLOCK:BMAL1-evoked E-box-mediated transcription.
KS15 modulates expression profiles of circadian clock genes as well as various cell-cycle regulators and pro-apoptotic genes. Moreover, KS15 modulates expression profiles of clock genes and cell-cycle- or apoptosis-related genes in MCF-7. KS15 can influence the expression of clock-controlled genes (CCGs) involved in the cell cycle and apoptosis.
KS15 exerts a dose-dependent inhibitory effect on the growth of MCF-7 cells, but no such effect on MCF-10A cells. Thus, KS15 can inhibit proliferation and increase the chemosensitivity of human breast cancer cells. KS15 can increase the activity of the CLOCK: BMAL1 heterodimer, thereby increasing the output of the molecular circadian clockwork. Furthermore, KS15 induces differential changes in cell cycle regulators and pro-apoptotic genes. KS15 inhibits MCF-7 cell growth and enhances susceptibility to anti-tumor drugs.
In conclusion, cryptochrome is a novel therapeutic target for the treatment of breast cancer, and that KS15 has potent anti-proliferative activity and increases the efficacy of chemotherapy in a specific type of human breast cancer.