Farnesyl transferase (FTase) is a zinc-dependent enzyme, which coordinates a zinc cation on its β subunit at the lip of the active site. FTase has two subunits: a 48kDa α-subunit and a 46kDa β-subunit. And it adds a 15-carbon isoprenoid called a farnesyl group to proteins bearing a CaaX motif. Ras-induced malignant transformation requires Ras farnesylation, a lipid posttranslational modification catalyzed by FTase. Furthermore, all Ras protein family members belong to a class of protein called small GTPase, and are involved in transmitting signals within cells. And there are four isoforms of the Ras protein exist: Ha-Ras, N-Ras, Ki-Ras 4A, and K-Ras 4B. Therefore, FTase inhibition is a potential mechanism to interfere with Ras-driven tumor growth.
Lonafarnib is a potent and orally active tricyclic farnesyl transferase (FTase) inhibitor.
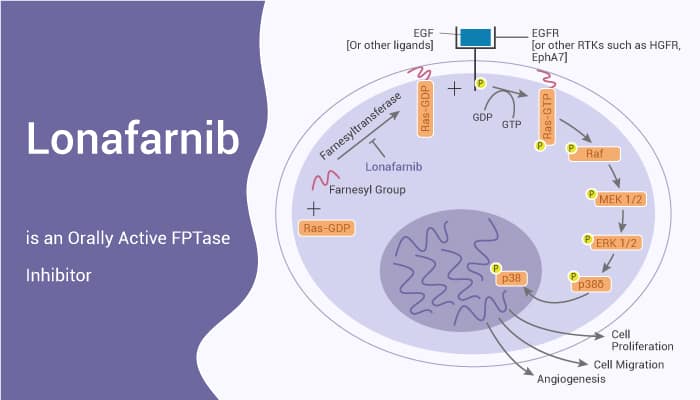
Lonafarnib, also known as SCH66336, can inhibit the activities of H-ras, K-ras and N-ras. In addition, Lonafarnib, which competes with the CAAX peptide substrate motif, blocks FTase activity in vitro. In cells, Lonafarnib potently inhibits Ha-Ras processing. Moverover, Lonafarnib blocks the transformed growth properties of fibroblasts and human tumor cell lines expressing activated Ki-Ras proteins. It also inhibits the anchorage-independent growth of many human tumor lines that lack an activated ras oncogene.
Lonafarnib has excellent pharmacokinetic properties in mouse, rat, and monkey. Furthermore, Lonafarnib shows potent anti-tumor activity in a wide array of human tumor xenograft models including tumors of colon, lung, pancreas, prostate, and urinary bladder origin. For example, in a Ha-Ras transgenic mouse model, prophylactic treatment with Lonafarnib delays tumor onset. And in a therapeutic mode, Lonafarnib treatment shows a significant tumor regression in the transgenic mice.
Additionally, Lonafarnib also can significantly reduce hepatitis delta virus (HDV) RNA levels.
To sum up, Lonafarnib is a potent and orally active farnesyl transferase inhibitor, and it inhibits the activities of H-ras, K-ras and N-ras.
References:
[1] M Liu, et al. Cancer Res. 1998 Nov 1;58(21):4947-56.
[2] Christopher Koh, et al. Lancet Infect Dis. 2015 Oct;15(10):1167-1174.