KIAA1718 is a histone demethylase for repressive methyl marks. what’s more, KIAA1718 involves in transcriptional activation. Specifically, KIAA1718, as a PHF2/PHF8 subfamily member, possesses histone demethylase activity specific for histone H3 lysine 9 (H3K9) and histone H3 lysine 9 (H3K27), transcriptionally repressive histone marks. Furthermore, KIAA1718 forms complexes with several factors including KAP1, a transcriptional co-activator. However, KIAA1718 belongs to a small family of Jumonji proteins with three members (PHF2, PHF8 and KIAA1718). Moreover, abnormal activities of these agent implicats in various pathological conditions like cancer. Here, we focus on the residue KIAA1718 Jumonji domain.
E67-2 is a selective KIAA1718 Jumonji domain inhibitor with low toxicity. To be specific, E67-2, as the E67 derivative, is a low-toxicity, selective KIAA1718 Jumonji domain inhibitor with an IC50 value of 3.4 µM. In vitro, E67-2 reduces inhibitory effect against G9a-like protein (GLP) by a factor of approximately 1500, resulting in an IC50 of 75 µM. Interestingly, E67-2 has much reduced inhibition on PHF8 on the doubly methylated H3(1-24)K4me3K9me2 peptide substrate. However, E67-2 has no effect on the activity against histone H3 lysine 4 (H3K4) demethylase JARID1C. Researches show that in the case of primary fibroblasts E67-2 has significantly reduced cell toxicity. Consequently, these show that a central role for KIAA1718 Jumonji Domain in cancer models systems and the selectivity of E67-2.
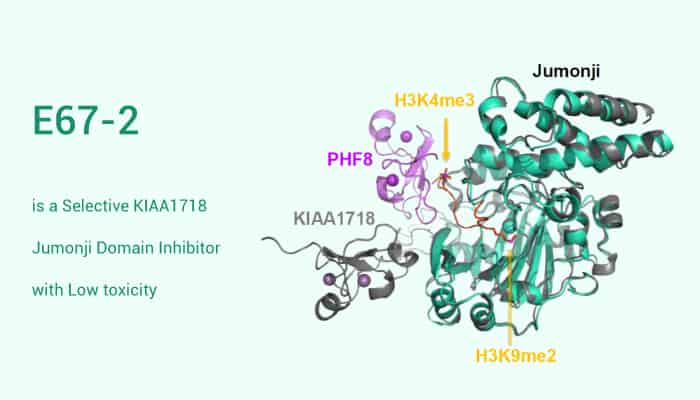
Taken together, E67-2 is a Selective KIAA1718 Jumonji Domain Inhibitor with Low toxicity. To sum up, E67-2 is a potent compound of cancer research.
References:
[1]. Upadhyay AK, et al. J Mol Biol. 2012 Feb 24;416(3):319-327.
[2]. Yokoyama A, et al. Genes Cells. 2010 Aug;15(8):867-873.