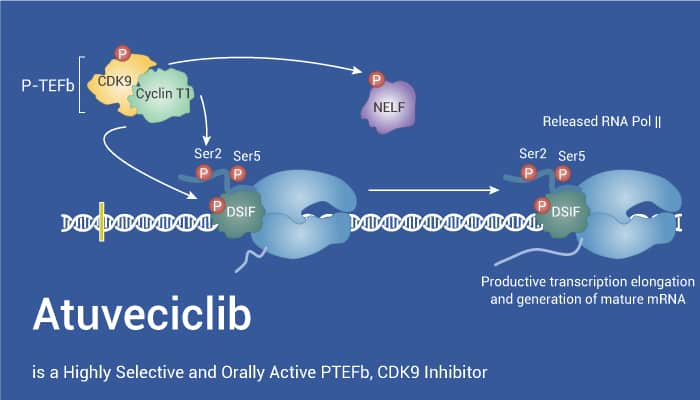
Positive transcription elongation factor P-TEFb is a kinase composed of CDK9 and cyclin T. Specifically, P-TEFb plays an important role in the transcriptional regulation of RNA polymerase II (pol II) in eukaryotes. Besides, P-TEFb mediates the positive transcriptional extension of the new mRNA chain by phosphorylating the S2 residue of YSPTSPS. YSPTSPS is repeated in series at the C-terminal domain (CTD) of RNA polymerase II (RNAP II). Moreover, P-TEFb is a cyclin-dependent kinase. It can phosphorylate DRB sensitivity inducer (DSIF) and negative elongation factor (NELF), as well as the carboxyl-terminal domain of Pol II large subunit. This leads to a shift to productive extension, resulting in mRNA synthesis.
Furthermore, CDK forms a functional complex with regulatory subunit (cyclin). Meanwhile, it is responsible for controlling cell proliferation, differentiation, apoptosis and DNA repair. CDK9 binds to cyclin T1, its main cyclin partner, to form P-TEFb. Nonetheless, CDK9 plays a key role in controlling global (non-ribosomal) transcription. Importantly, CDK9 is relevant to a variety of physiological processes besides transcription, including differentiation, apoptosis, and signal transduction. Therefore, enhanced CDK9 activity leads to poor prognosis in many cancer types. Here, we will introduce a highly selective, oral PTEFb/CDK9 inhibitor, Atuveciclib.
Atuveciclib is a Highly Selective and Orally Active PTEFb, CDK9 Inhibitor.
First of all, Atuveciclib (BAY-1143572) inhibits CDK9/CycT1 with an IC50 of 13 nM. Particularly, PTEFb is a heterodimer of CDK9 and one of four cyclin partners, cyclin T1, cyclin K, cyclin T2a or cyclin T2b. Obviously, Atuveciclib demonstrates potent antiproliferative activity against HeLa cells (IC50=920 nM) and MOLM-13 cells (IC50=310 nM).
In the second place, In vivo efficacy studies in the MOLM-13 xenograft model in mice, Atuveciclib demonstrates high antitumor efficacy. Interestingly, Atuveciclib with 6.25 or 12.5 mg/kg/day results in a dose-dependent antitumor efficacy. It has a treatment-to-control (T/C) ratio of 0.64 and 0.49, respectively. In fact, the 25 mg/kg once daily dose is the maximum tolerated dose in nude mice. Furthermore, Atuveciclib with 25 or 35 mg/kg, three days on/two days off, results in a T/C ratio of 0.33 and 0.20, respectively. In an in vivo pharmacokinetic study in rats, Atuveciclib shows low blood clearance (CLb 1.1 L/kg per hour).
All in all, Atuveciclib is a potent and highly selective, oral PTEFb/CDK9 inhibitor.
References:
Lücking U, et al. ChemMedChem. 2017 Nov 8;12(21):1776-1793.