Aminopeptidases catalyze the cleavage of amino acids from the amino terminus of protein or peptide substrates. Regarding catalytic mechanism, most of the aminopeptidases are metallo-enzymes. In addition, aminopeptidase is widely present in animal and plant kingdomsis widely present in , found in many subcellular organelles, in cytoplasm, and as membrane components. Several aminopeptidases perform essential cellular functions. CD13/APN (aminopeptidase N) is a selective epitope first identified by phage display peptide assays in the tumor vasculature and in hypoxia-induced retinal neovessels.
Bestatin (also known as Ubenimex) is a natural, broad-spectrum, and competitive aminopeptidase and leukotriene A4 hydrolase (LTA4H) inhibitor. In addition, Bestatin inhibits CD13/APN (aminopeptidase N), which is a selective angiogenic marker expressed in tumor vasculature. Partha S. Bhattacharjee et al. demonstrated that Bestatin inhibits blocks inflammatory leukostasis and blood-retinal barrier permeability through downregulation inflammatory mediators of extracellular matrix (ECM) degradation and angiogenesis in the diabetic retina. Moreover, Bestatin is also an immunomodulating agent in anti-leukemia treatment. The mechanism by which Bestatin enhances all-trans retinoic acid (ATRA)-induced cell differentiation of acute promyelocytic leukemia (APL) cells is generally attributed to inhibition of cell surface CD13/aminopeptidase N activity. Indeed, Bestatin enhances ATRA-induced differentiation and inhibits ATRA-driven phosphorylation of p38 MAPK in ATRA-sensitive APL NB4 cells. On the contrary, Bestatin does not reverse the differentiation block in ATRA-resistant APL MR2 cells, in which ATRA is unable to induce phosphorylation of p38 MAPK.
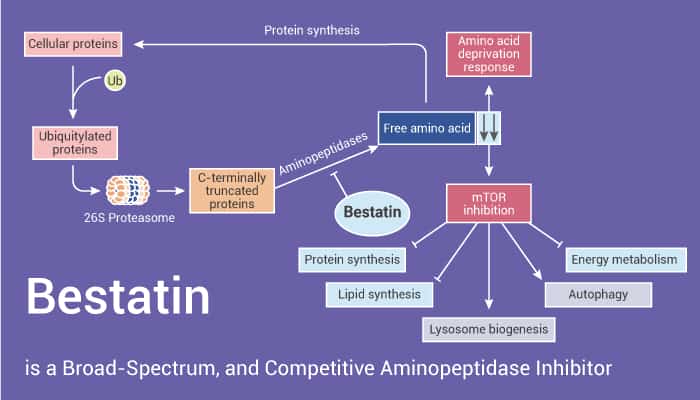
To sum up, Bestatin is a broad-spectrum and competitive aminopeptidase and LTA4H inhibitor, with anticancer effects.
References:
[1] Ahamed Hossain, et al. Exp Eye Res. 2016 Aug;149:100-106.
[2] Xijun Qian, et al. Am J Ther. May-Jun 2016;23(3):e680-9.