Indoleamine 2,3-dioxygenase (IDO) is an enzyme of interest in immuno-oncology. It has immunosuppressive effects that result from its role in tryptophan catabolism. IDO1 catalyzes the initial rate-limiting step in the degradation of the essential amino acid tryptophan along the kynurenine pathway. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR. IDO1 enzyme also relates directly to the modulation of the innate and adaptive immune system. IDO1 has received a great deal of attention as a driver of tumor-mediated suppression. It is active in many human cancers and its expression has been associated widely with poor prognosis. Accordingly, inhibitors of the enzymatic activity and effector functions of IDO1 are the tools to leverage cancer therapy. In this study, Indoximod (1-Methyl-D-tryptophan) is an orally active IDO pathway inhibitor.
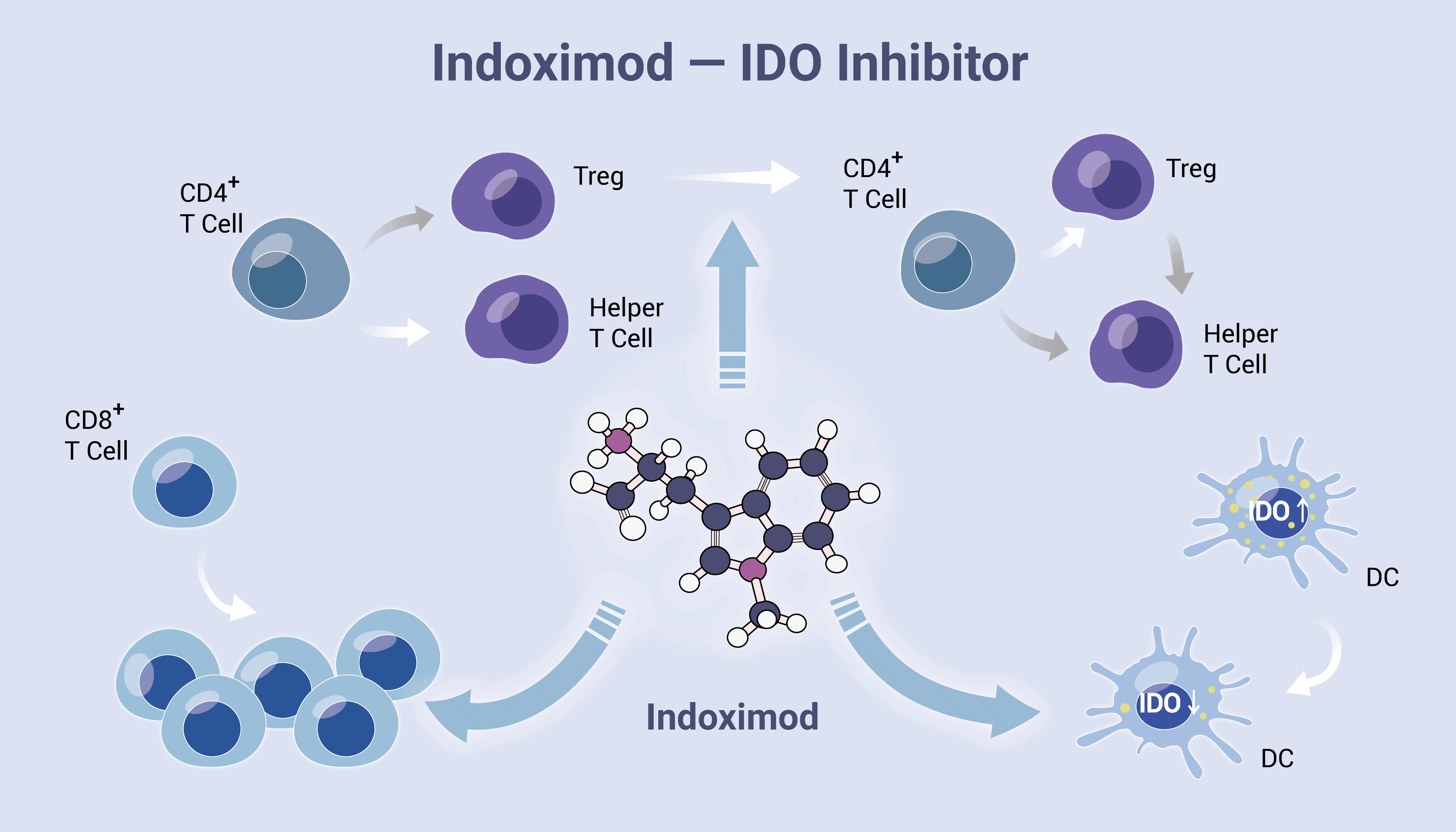
In contrast to direct enzymatic inhibitors of IDO1, Indoximod acts downstream of IDO1 to stimulate mTORC1. mTORC1 is a convergent effector signaling molecule for all IDO/TDO enzymes, thus possibly lowering the risks of drug resistance by IDO1 bypass. D-1MT activates a Trp sufficiency signal that stimulates mTOR but does not affect GCN2. In a word, Indoximod is a Trp mimetic which mTORC1 interprets as L-Trp under conditions of high Trp catabolism and autophagy. Thus, the drug acts to block the suppressive signal on the mTORC1 function generated by IDO/TDO activity. Indoximod lies at a leading edge of broad-spectrum immunometabolic agents that may act to improve responses to many anticancer modalities, in a manner analogous to vaccine adjuvants that act to boost immunity in settings of infectious disease.
Indoximod is an IDO pathway inhibitor. It acts as a Trp mimetic in regulating mTOR. Furthermore, Indoximod supports immunotherapeutic vaccines susceptibility and tumor-specific targeting by reducing tumorigenesis signaling pathways.
Reference:
Fox E, et al. Front Oncol. 2018;8:370. Published 2018 Sep 11.