Antibody-dependent cytotoxicity (ADCC) is a cell-mediated immune defense mechanism. The effector cells of the immune system actively cleave target cells, and their membrane surface antigens are bound by specific antibodies. Besides, it is one of the mechanisms by which antibodies, as part of the humoral immune response, can limit and contain the infection. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) is a member of the tumor necrosis factor (TNF) family of cytokines. Moreover, it has proangiogenic and proinflammatory properties in vivo and induces cell death in tumor cell lines. Here, we will introduce a humanized IgG1 monoclonal antibody targeting TWEAK, Enavatuzumab.
The TWEAK effect is mediated by the membrane receptor Fn14. TWEAK binds to Fn14 and controls many cellular activities, including proliferation, migration, differentiation, apoptosis, angiogenesis, and inflammation. Meanwhile, TWEAK-induced TRAF2 translocation is accompanied by nuclear factor kappa B 2 (NF-κB2)/p100 and is processed into p52 with a strong increase. This indicates that TRAF2 redistribution is sufficient to activate alternative NF-κB way. In addition, TWEAK-induced cells are sensitive to TNFR1-mediated apoptosis and necrosis cell death. TWEAK through Fn14 and IκB kinase stimulates the Transcriptional activity of NF-κB. TWEAK/Fn14 interaction is particularly important in synovitis relevant to rheumatoid arthritis (RA).
Enavatuzumab (PDL192) is a Humanized IgG1 Monoclonal Antibody Targeting TWEAK.
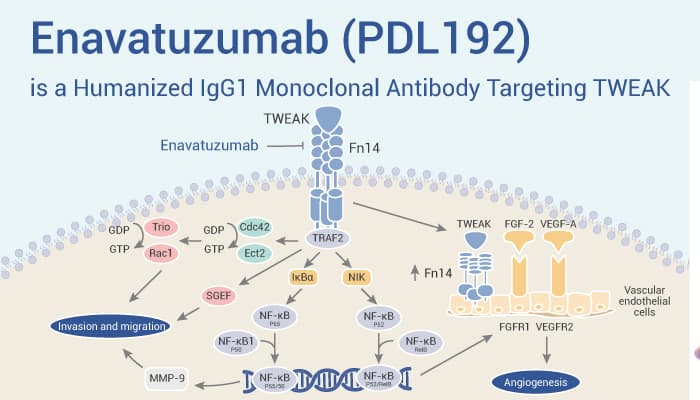
At first, TWEAK (Fn14; TNFRSF12A), the natural ligand of the TWEAK receptor (TweakR), stimulates multiple cellular responses. Importantly, Enavatuzumab induces tumor growth inhibition through direct TweakR signaling and antibody-dependent cell-mediated cytotoxicity (ADCC). Particularly, Enavatuzumab can actively recruit and activates myeloid effectors to kill tumor cells. Obviously, Enavatuzumab inhibits the growth of various human TweakR-positive cancer cell lines and xenografts in vitro and in vivo.
Secondly, Enavatuzumab with 0.1-1000 ng/mL for 4 hours induces effector cell activation and tumor cell killing in vitro. Enavatuzumab results in significantly increased migration of immune effector cells toward the tumor cells in SN12C and A375 cells.
Thirdly, Enavatuzumab with 10 mg/kg by IP three times per week for 7 doses shows diverse antitumor activities on different xenograft tumors. Additionally, Enavatuzumab up-regulated the activation markers on splenocytes in SN12C tumor-bearing mice.
Finally, Enavatuzumab (PDL192; ABT-361) is a humanized IgG1 monoclonal antibody targeting the receptor of TWEAK.
References:
Shiming Ye, et al. J Immunol Res. 2017;2017:5737159.