Ovarian cancer (OC) is the eighth most common neoplasia in women, and the fifth leading cause of cancer-related deaths. Due to a lack of early stage detection, about 70% of affected patients, present with advanced disease (FIGO stage II–IV) at the time of diagnosis. Thus OC becomes the deadliest of gynecological cancers. As the most intensively studied group of antiblastic agents for the management of recurrent ovarian cancer, Poly-ADP-Ribose Polymerase (PARP) inhibitors gets more and more attention. According to study, PARP-1 is the most abundant of a family of PARP enzymes. PARP-1 together with PARP-2 (and to a limited extent PARP-3) is responsible for facilitating DNA repair. Therefore, PARP-1 inhibitor inhibits repair and increase the antitumour activity of DNA-damaging chemotherapy and radiotherapy. Besides, Olaparib was the first to be approved by European Medicines Agency as maintenance therapy post-response to platinum-based chemotherapy. Moreover, Olaparib has good potential of recurrent ovarian cancer in women with deleterious BRCA1/2 mutation. Hence, we will introduce the PARP inhibitor-Rucaparib (AG014699; PF-01367338).
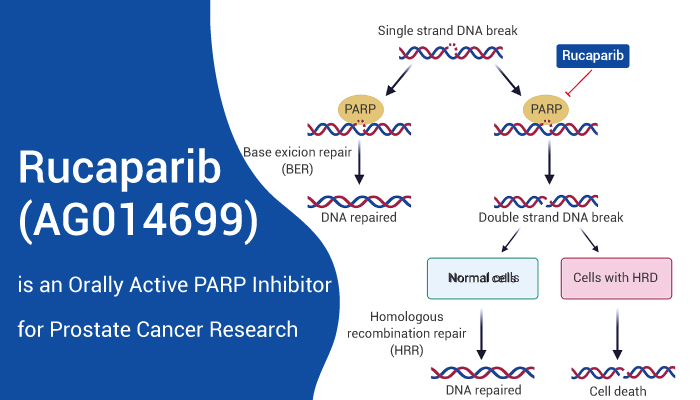
Rucaparib is an orally active PARP-1/2/3 inhibitor (Ki=1.4 nM for PARP1).
Rucaparib (0.1, 1, 10, 100 μM; 24 h) is cytotoxic, and has the LC50 being 5 μM in Capan-1 (BRCA2 mutant) cells and only 100 nM in MX-1 (BRCA1 mutant) cells. In addition, Rucaparib inhibits PARP-1 activity by 97.1% at a concentration of 1 μM in permeabilised D283Med cells.
Rucaparib also shows good activities in vivo. Rucaparib (10 mg/kg for i.p. ; 50, 150 mg/kg for p.o.; daily for 5 days per week for 6 weeks) significantly inhibits the growth of the tumor. Besides, Rucaparib (0.1 mg/kg) also results in a 50% increase in the temozolomide-induced tumor growth delay. Moreover, Rucaparib (150 mg/kg; p.o.; single weekly or 3 times per week for 6 weeks) has greatest antitumor effect with three complete regressions.
Reference:
[1] Musella A, et al. Cancer Treat Rev. 2018 May;66:7-14.
[2] Thomas HD, et al. Mol Cancer Ther. 2007 Mar;6(3):945-56.
[3] Murray J, et al. Br J Cancer. 2014 Apr 15;110(8):1977-84.
[4] Shirley M. Target Oncol. 2019 Apr;14(2):237-246.