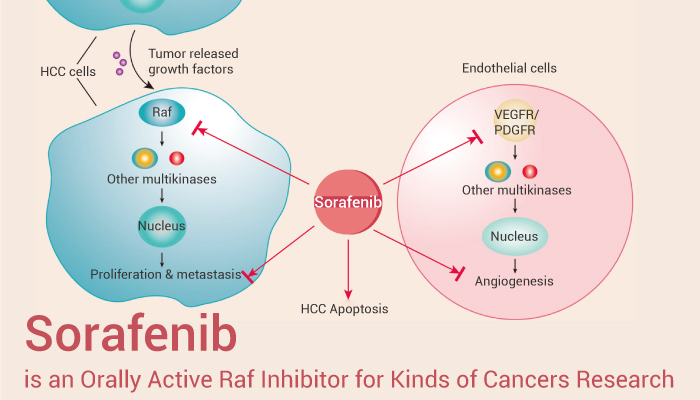
Sorafenib is an oral multi-kinase inhibitor that inhibits cell proliferation through a strong inhibition of the serine/threonine kinase RAF. Sorafenib was initially approved by the FDA for treatment of metastatic renal cell carcinoma, and later it was approved for management of HCC and metastatic differentiated thyroid carcinoma. Moreover, it was shown to inhibit pro-angiogenic VEGF and platelet-derived growth factor receptor.
Sorafenib is a potent and orally active Raf inhibitor for cancer research
First of all, Sorafenib (Bay 43-9006) is a potent and orally active Raf inhibitor with IC50s of 6 nM and 20 nM for Raf-1 and B-Raf, respectively. Meanwhile, Sorafenib is a multikinase inhibitor with IC50s of 90 nM, 15 nM, 20 nM, 57 nM and 58 nM for VEGFR2, VEGFR3, PDGFRβ, FLT3 and c-Kit, respectively. Furthermore, in MDA-MB-231 breast cancer cells, Sorafenib completely blocks activation of the MAPK pathway. Finally, Sorafenib (0.01 to 3 μM) has dose-dependent inhibition of basal MEK1/2 and ERK1/2 phosphorylation with IC50 values of 40 and 100 nM, respectively.
In vivo: Sorafenib demonstrates broad oral antitumor efficacy in panel of human tumor xenograft models. First, Sorafenib (7.5 to 60 mg/kg, oral) has no lethality and no increase in weight loss in any treated group relative to the corresponding control group. Second, Sorafenib (30 to 60 mg/kg, oral, daily) produces complete tumor stasis during treatment in five of the six models. Third, Sorafenib group has 83.3 % survival rate compared to 100 % in the normal control group. Moreover, the liver index in Sorafenib group significantly decreases to lower than its value in the normal control.
In a word, Sorafenib has anti-tumor activity. In addition, Sorafenib can induce autophagy and apoptosis. Sorafenib also is a ferroptosis activator.
Reference:
[1] Daher S, et al. J Clin Transl Hepatol. 2018 Mar 28;6(1):69-78.
[2] Wilhelm SM, et al. Cancer Res. 2004 Oct 1;64(19):7099-109.
[3] Jin W, et al. Oncol Rep. 2017 Jan;37(1):273-280.