The MYC family of oncogenes (MYC, MYCN, and MYCL), encodes basic helix-loop-helix leucine zipper (bHLHZip) transcription factors. Through the HLHZip domain, MYC heterodimerizes with the bHLHZip protein MAX, which enables the MYC:MAX complex to bind E-box regulatory DNA elements throughout the genome. Therefore, it controls the transcription of a large group of specific genes. The direct target gene products, in turn, influences global RNA and protein synthesis, thereby coordinating multiple fundamental cellular processes, including cell cycle progression, cell growth, apoptosis, senescence, metabolism and stem cell functions. MYC is one of the most important drivers of tumor development and has been highlighted as a key therapeutic target for cancer therapy for a number of tumor types. MYC is strictly dependent on MAX for binding E-boxes, targeting MYC:MAX interaction is a conceivable approach to target MYC. In this study, MYCMI-6 is a potent and selective endogenous MYC:MAX protein interactions inhibitor.
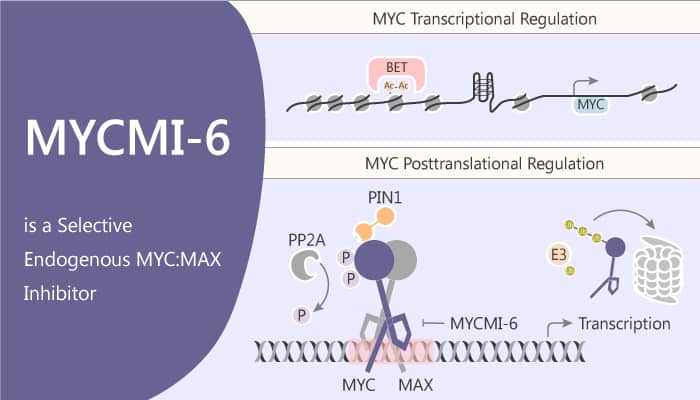
MYCMI-6 blocks MYC-driven transcription binds selectively to the MYC bHLHZip domain and induces apoptosis.
MYCMI-6 blocks MYC-driven transcription and binds selectively to the MYC bHLHZip domain with a Kd of 1.6 μM. It inhibits tumor cell growth in an MYC-dependent manner (IC50<0.5 μM). Moreover, it significantly inhibits the growth of Burkitt’s lymphoma cells (Mutu, Daudi, and ST486), having translocations of MYC to one of the immunoglobulin loci – in a dose-dependent manner with an average GI50 of 0.5 μM. Further, the treatment of MCF7 cells with the MYCMI-6 for 24 hours significantly decreases MYC:MAX is PLA signals to 7%. Titration shows an IC50 for inhibition of MYC:MAX of less than 1.5 μM for MYCMI-6 by PLA. MYCMI-6 inhibits the MYC:MAX heterodimer formation with an IC50 of 3.8 μM. In addition, MYCMI-6 efficiently inhibits anchorage-independent growth of MYCN-amplified neuroblastoma cells with GI50 values of <0.4 μM.
In addition, MYCMI-6 induces massive apoptosis and reduces tumor cell proliferation, tumor microvasculature density, and MYC:MAX interaction in an MYC-dependent xenograft tumor model. However, For future research, it is important to determine the precise mechanism of action of MYCMI-6. Thus, it enables to develop more potent and selective molecules towards clinically relevant direct MYC inhibitors.
Reference:
Castell A, et al. Sci Rep. 2018 Jul 3;8(1):10064.