According to the World Health Organization (WHO), Glioblastoma is the most common primary brain neoplasm. It comprises 15% of all intracranial neoplasms and 60-75% of astrocytic tumors. Unfortunately, Glioblastoma is the most aggressive and prevalent primary malignant brain tumor of the central nervous system. Glioblastoma derives specifically from the astrocyte cell type, in which the cellular growth is unregulated and tumor formation occurs.
In this study, Yufeng Shi, et al find that Gboxin is an oxidative phosphorylation inhibitor. Gboxin isolates from a high-throughput chemical screen based on low-passage primary glioblastoma (GBM) cells. Gboxin targets unique features of mitochondrial pH in GBM and other cancer cells. As a result, Gboxin exerts its tumour-cell-specific toxicity in primary culture and in vivo.
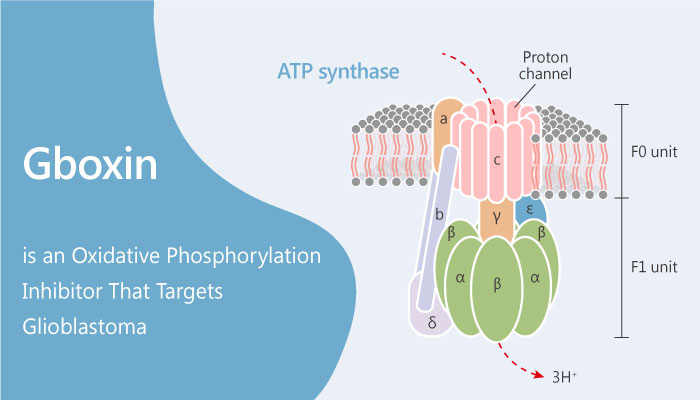
Oxidative phosphorylation is a process of a series of energy transformations. These effects are cellular respiration or simply respiration in their entirety. Oxidative phosphorylation (OXPHOS) is an electron transfer chain driven by substrate oxidation. This effect accompanies the synthesis of ATP through an electrochemical transmembrane gradient. Gboxin inhibits the activity of F0F1ATP synthase. Besides, Gboxin specifically inhibits the growth of primary mouse and human glioblastoma cells. However, Gboxin does not inhibit the growth of mouse embryonic fibroblasts or neonatal astrocytes. Moreover, Gboxin rapidly and irreversibly compromises oxygen consumption in glioblastoma cells. Gboxin disrupts primary glioblastoma cell metabolism. Besides, Gboxin also causes acute oxygen consumption rate (OCR) depletion in mouse embryonic fibroblasts (MEFs) and astrocytes. Gboxin causes an irreversible inhibition of oxidative phosphorylation (OXPHOS) in high-throughput GBM sphere (HTS) cells but not in wild-type cells. In conclusion, Gboxin is an oxidative phosphorylation inhibitor that targets glioblastoma.
Reference: