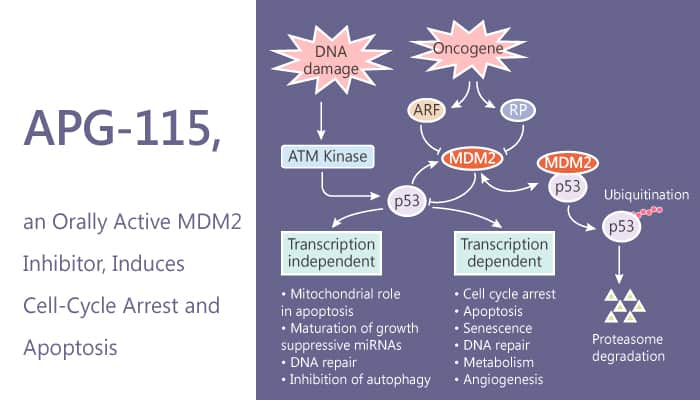
The essential all human cancers compromises the tumor suppressor function of p53. In about half of human cancers, TP53, the gene encoding p53 protein, is mutated or deleted. This inactivates the tumor suppressor function of p53. In the remaining 50%, p53 retains its wild-type status. But, murine double minute 2 (MDM2) protein effectively inhibits its function through a direct protein–protein interaction. Restoring p53 function by inhibiting its interaction with MDM2 is a promising therapeutic strategy for cancer. APG-115 is a potent, orally active MDM2 inhibitor. It binds to MDM2 protein with IC50 and Ki values of 3.8 nM and 1 nM. Moreover, it blocks the interaction of MDM2 and p53.
In vitro, APG-115 inhibits cell proliferation in concentration-dependent manner, with IC50s of 18.9 ± 15.6 nM and 103.5 ± 18.3 nM respectively in AGS and MKN45 cells. It enhances the anti-proliferative effect of radiotherapy at different radiation dose. Moreover, APG-115 affects progression by inducing cells arrested at G0/G1 phase in AGS and MKN45 cell with wild p53.
It activates p53 to enhance radiosensitivity in AGS and MKN45 cells. In addition, APG-115 leads to a concentration-dependent cell cycle arrest in G2/M phases and a decreasing in S-phase in p53 wide-type cell lines (TPC-1, KTC-1).
In vivo, APG-115 significantly suppresses the growth of gastric xenograft tumors. Meanwhile, it significantly enhances anti-tumor effect by radiation.
All in all, APG-115 significantly inhibits cell viability, induces cell cycle arrest at G1 phase. Furthermore, it enhances radiosensitivity of gastric cancer. However, APG-115 combines with radiation needs further clinical trial to confirm the anti-tumor effect in gastric.
Reference:
Hanjie Yi ea al, J Exp Clin Cancer Res. 2018 May 2;37(1):97.