Nuclear factor (erythroid-derived 2)-like 2 (NRF2) is a master regulator of the cellular antioxidant response. It is genetically activated in Non-Small Cell Lung Cancers (NSCLCs) by, for instance, mutations in the interacting protein KEAP1. Its aberrant activation rewires biochemical networks in cancer cells that may create special vulnerabilities. KEAP1-mutant NSCLC cells selectively express druggable proteins. NR0B1 is an atypical orphan nuclear receptor. In this study, they show engages in a multimeric protein complex to regulate the transcriptional output of KEAP1-mutant NSCLC cells. The compounds covalently target a conserved cysteine within the NR0B1 protein interaction domain. Furthermore, these compounds disrupt NR0B1 complexes and impair the anchorage-independent growth of KEAP1-mutant cancer cells. BPK-29 is a specific ligand that disrupts the NR0B1 protein-protein interactions by covalently modifying C274.
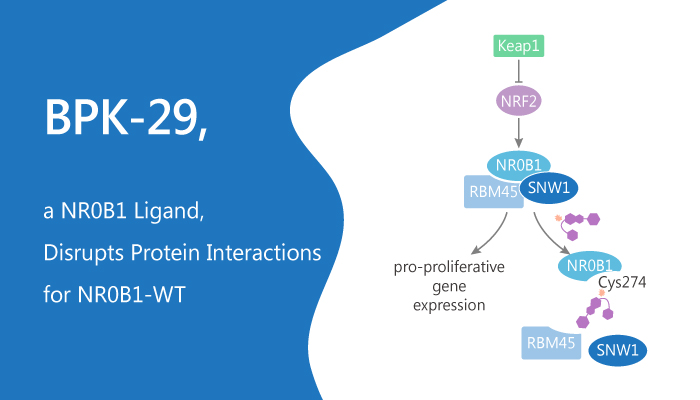
BPK-29yne probe characterizes the protein targeting of BPK-29 in NSCLC cells. it suggests that BPK-29 substantially engage NR0B1 with good overall proteomic selectivity in KEAP1-mutant NSCLCs. Meanwhile, BPK-29 blocks the interactions of FLAG-tagged RMB45 or SNW1 with endogenous NR0B1. BPK-29 blocks NR0B1-protein interactions with good potency. Treatment of KEAP1-mutant NSCLC cells with BPK-29 (5 μM) blocks colony formation in soft agar. BPK-29 also produces some of the gene expression changes caused by shRNA-mediated disruption of NR0B1 or NRF2 in KEAP1-mutant NSCLC cells. They include reductions in CRY1, DEPDC1, and CPLX2 . However, treatment of BPK-29 do not observe this in KEAP1-WT NSCLC cells. Furthermore, BPK-29-treated cells also show a substantial reduction in CRY1 protein content.
In summary, NR0B1 lacks a clear ligand-binding pocket, this protein may act primarily as a coregulatory protein to affect transcription. BPK-29 disrupts the NR0B1 protein-protein interactions and impairs the anchorage-independent growth of KEAP1-mutant cancer cells.
Reference:
Bar-Peled L, et al. Cell. 2017 Oct 19;171(3):696-709.e23.