The Ras/Raf/MEK/ERK signaling cascade integrates extracellular clues from cell surface receptors to gene expression and regulation of multiple cellular proteins. Extracellular-signal-regulated kinases (ERKs) serve as key central nodes within this pathway. Simultaneous targeting of multiple effectors such as RAF, MEK, and ERK offers the potential for enhanced efficacy while delaying and overcoming resistance. In this study, Shripad V. Bhagwat, et al report the characterization of LY3214996.
ERK cascade plays a crucial role in multiple cellular processes such as cell proliferation, differentiation, adhesion, migration, and survival. LY3214996 is a highly selective inhibitor of ERK1 and ERK2, with IC50 of 5 nM for both enzymes in biochemical assays. Meanwhile, LY3214996 potently inhibits cellular p-RSK1 in BRAF and RAS mutant cancer cell lines. AGO1 is a major component of RNA-induced silencing complexes and plays a crucial role in solid tumors. It significantly inhibits the growth and migration of the AGO1 cells.
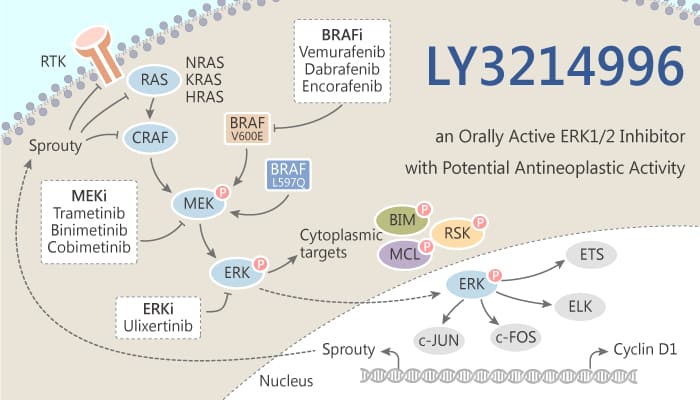
In tumor xenograft models, LY3214996 inhibits pharmacodynamic biomarker p-p90RSK1 in tumors. Oral administration of single-agent LY3214996 significantly inhibits tumor growth in vivo and is well tolerated in BRAF or NRAS mutant melanoma, BRAF or KRAS mutant colorectal, lung and pancreatic cancer xenografts or patient-derived xenografts models. In addition, LY3214996 has anti-tumor activity in a Vemurafenib-resistant A375 melanoma xenograft model due to MAPK reactivation. More importantly, combination treatment of LY3214996 (ERK inhibitor) and CDK4/6 inhibitor Abemaciclib is well tolerated and results in potent tumor growth inhibition or regression in multiple in vivo cancer models, including KRAS mutant colorectal and non-small cell lung cancers.
In summary, LY3214996 is a selective and novel ERK1/2 inhibitor with potent antitumor activities in cancer models with MAPK pathway alterations.