Tumor cells use a variety of oncogenically- and environmentally driven metabolic pathways. That aims to meet the bioenergetic and biosynthetic demands of rapid and sustained growth. One of the key nutrients is the amino acid glutamine. It is the most abundant amino acid in plasma. Small-molecule inhibitors, suppressing the broadly expressed form of glutaminase (encoded by the gene GLS), has antitumor activity across a variety of tumor types. They include lymphoma, glioma, breast, pancreatic, non-small cell lung and renal cancers. In addition, elevated GLS expression is associated with high grade and metastatic breast cancer. In particular, triple-negative breast cancer (TNBC) primary tumors and cell lines have elevated GLS mRNA; this is associated with high glutamine consumption and/or enhanced reliance on exogenous glutamine for survival in vitro. In this study, Telaglenastat is a first-in-class, reversible and orally bioavailable glutaminase 1 (GLS1) inhibitor.
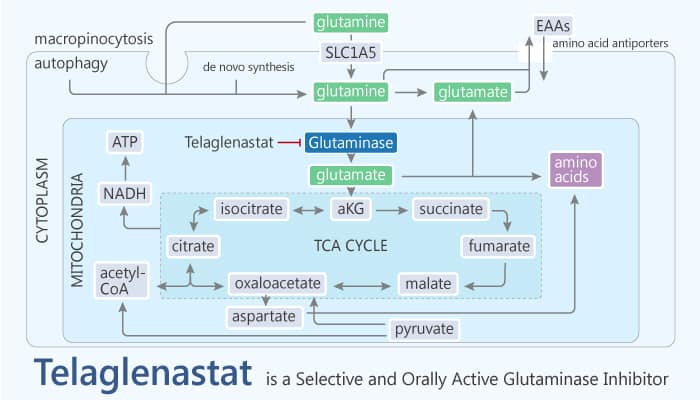
Telaglenastat shows high selectivity for the GLS1 splice variants KGA (kidney-type glutaminase) and GAC (glutaminase C) over GLS2. The IC50s are 23 and 28 nM for endogenous glutaminase in mouse kidney and brain, respectively. In addition, Telaglenastat inhibits the two TNBC cell lines with IC50 ranging from 20 to 55 nM. Furthermore, it exhibits in vitro antiproliferative activity against a panel of TNBC cell lines. Importantly, Telaglenastat inhibits glutamine consumption in HCC1806 cells. Moreover, TNBC cell lines are sensitive to glutaminase inhibition with Telaglenastat. Telaglenastat activates caspase 3/7 and induces apoptosis in MDA-MB-231 and HCC1806 cells. In addition, it has antitumor activity in xenograft models of TNBC.
In summary, Telaglenastat is a potent, selective, and orally bioavailable inhibitor of both splice variants of glutaminase (KGA and GAC). Thus, Telaglenastat is a targeted therapeutic in TNBC and other glutamine-dependent tumors.
Reference:
Gross MI, et al. Mol Cancer Ther. 2014 Apr;13(4):890-901.