Cancer cells regularly acquire resistance to programmed cell death, or apoptosis, which not only supports cancer progression but also leads to resistance to therapeutic agents.
In this study, researchers focus their research on the inhibitor of apoptosis protein (IAP) family. IAPs are a disparate group of proteins that all contain a baculovirus IAP repeat domain, which is important for the inhibition of apoptosis in some, but not all, family members. Especially, SBP-0636457 is a SMAC mimetic, and as an IAP antagonist.
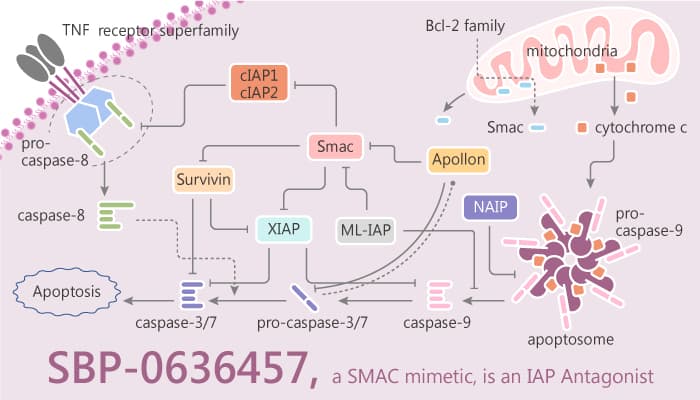
IIAP family is a functionally and structurally related group of proteins that serve as endogenous inhibitors of programmed cell death, or apoptosis. In addition, IAPs are targets for anti-cancer therapeutics. The first human IAP revealed is neuronal apoptosis inhibitory protein (NAIP or BIRC1). The next human IAPs are the cellular IAPs 1 and 2: cIAP1 (or BIRC2) and cIAP2 (or BIRC3). These proteins have a role in tumor necrosis factor receptor signaling through association with the adaptor proteins TRAF1 and TRAF2.
Several proteins share the common BIR domain, which leads to the identification of more family members via traditional homology-matching database searches. In particular, BIR domains are zinc finger domains and invariantly contain three cysteines and one histidine, which co-ordinate the zinc ion, and these domains are involved in various protein-protein interactions (PPIs).
Notably, IAP inhibitors (Smac mimetics) act as potential anti-cancer agents. SBI-0636457 is a small-molecule Smac mimetic and potentiates TNF-induced and TRAIL-induced apoptosis. Moreover, SBI-0636457 has potent cell-killing effects in several subtypes of breast, ovarian, and prostate cancer cell lines. Furthermore, SBI-0636457 administeres as a single agent exhibited no toxicity to normal human fibroblasts.