The family of caveolin membrane proteins plays many roles in the cell’s structural, signaling, and transportation processes. Caveolin-1 (CAV) is a monotonic membrane protein, 22 kDa, it penetrates only one leaflet of the lipid bilayer, and both the N- and C-termini remain on the cytoplasmic side. Multiple copies of CAV oligomerize can form high molecular weight complexes that bend the membrane inward to form invaginations, termed “caveolae,” of 50-100 nm in diameter.
In this article, we will introduce a molecular tool to disrupt oligomerization caveolin-1 (CAV), WL47.
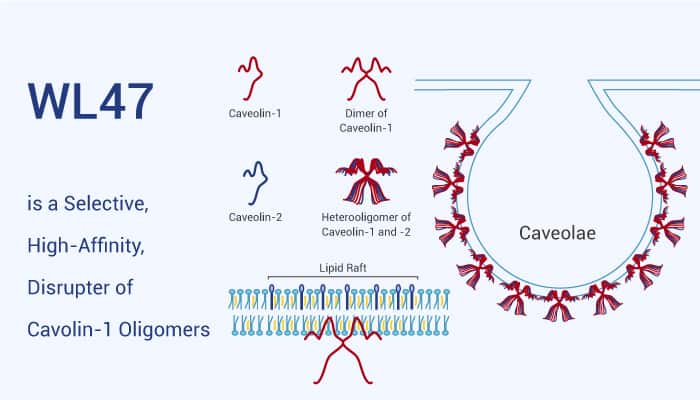
WL47 is a high-affinity caveolin-1 (CAV1) ligand (Kd=23 nM). Additionally, it is also a potent disrupter of CAV1 oligomers. WL47 shows selectivity for CAV1 over BSA, casein, and HEWL. Whatmore, this compound is 80% smaller in length than the original T20 parent sequence and can be used for the study of caveolin-1 function.
Previous researches show that the interaction between CAV and gp41 is a ) acts as a starting point for generating a ligand for CAV. T20 is a 36 amino acid peptide derived from gp41 and blocks HIV viral fusion with CD4+ T-cells. WL47 is 80% smaller in length and has 7500-fold greater affinity than the original T20 parent sequence.
In vitro, Demonstrating WL47 activity with CAV oligomers and a method for measuring the degree of oligomerization. A variant of CAV (CAV(FLV)) that spontaneously oligomerizes to form CAV nanoparticles with diameters is used to examine deoligomerization by WL47.
WL47 effectively disrupts these nanoparticles, but it does not disrupt oligomerization in the presence of a reducing agent. This demonstrating that WL47 playing its roles requires dimerization by a disulfide bond.
In summary, this CAV ligand, WL47, facilitates future research by providing a mechanism for perturbing the CAV function. It also provides a potential lead compound for a new type of therapeutics.
Reference:
Amanda J H Gilliam, et al. J Med Chem. 2016 Apr 28;59(8):4019-25.