Diacylglycerol kinase (DGK) is a second messenger in its own right and can modulate the activity of PKC and several ion channels. Chemokines are low MW extracellular signaling peptides secreted by tissue either constitutively during homeostasis or de novo during an inflammatory response. They are a diverse family of peptides (CC, CXC, CX3C, and XC chemokines). They have a key role in the tissue recruitment and interstitial migration of leukocytes and other cell types, as well as acting both as chemoattractants and as cues for cellular arrest.
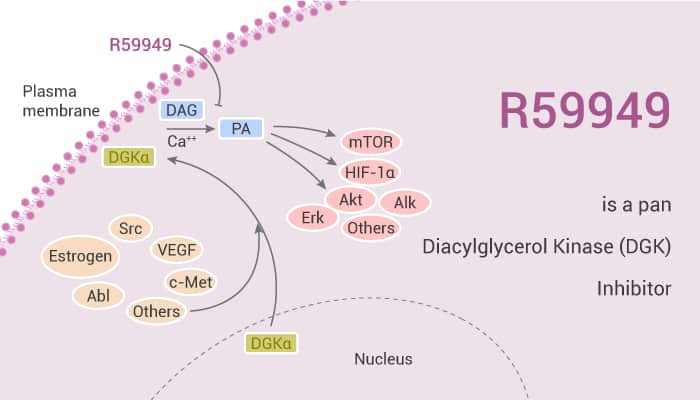
CCL2 is an inflammatory chemokine that stimulates the recruitment of monocytes into tissue via activation of the GPCR CCR2. Pharmacological inhibition of DAG metabolism is a novel route to attenuate CCL2 signaling in human monocytes. In this study, R59949 is a pan DGK inhibitor with an IC50 of 300 nM.
R59949 is a DGK inhibitor. It also strongly inhibits the activity of type I DGK α and γ. Moreover, it moderately attenuates the activity of type II DGK θ and κ. Moreover, R59949 activates protein kinase C (PKC) by enhancing the levels of the endogenous ligand diacylglycerol. CCL2 evokes intracellular Ca2+ signals and stimulates the migration in THP-1 monocytic cells and human CD14+ monocytes in a CCR2-dependent fashion. In THP-1 monocytes, R59949 attenuates CCL2-evoked Ca2+ signaling with a half-maximal concentration of 8.6 μM. Furthermore, R59949 inhibits inducible NO production through decreasing transplasmalemmal L-arginine uptake.
In summary, the inhibition of DGK and DAG lipase is a novel pharmacological route. It can suppress CCL2-mediated intracellular Ca2+ signaling and migration in human monocytes.
Reference:
Day P, et al. Br J Pharmacol. 2019;176(15):2736-2749.