CDKs remain attractive targets for anti-proliferative cancer chemotherapy. they play a central role in cell division and growth. Amongst the cell cycle CDKs, CDK2 has attracted the most attention and its exploitation as a drug target based on structure-based drug design.
In this article, we will introduce a potent CDK1 and CDK2 inhibitor, NU6102.
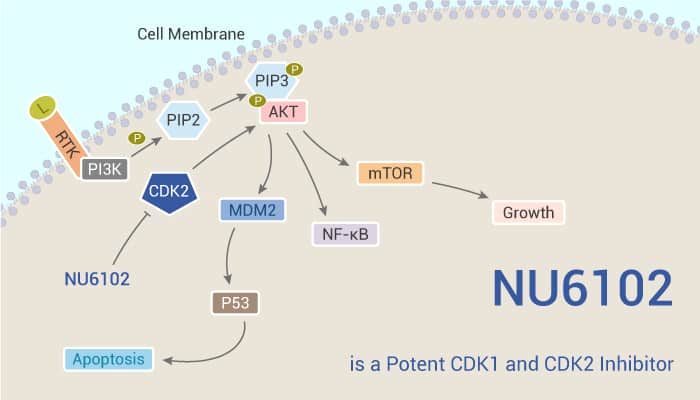
This inhibitor exhibits IC50s of 9.5 nM and 5.4 nM for CDK1/cyclin B and CDK2/cyclinA3, respectively. Additionally, NU6102 shows selectivity for CDK1/CDK2 over CDK4 (IC50 of 1.6 μM), DYRK1A (IC50=0.9 μM), PDK1 (IC50=0.8 μM) and ROCKII (IC50=0.6 μM).In SKUT-1B cells, NU6102 treatment induces a G2 arrest and inhibition of Rb phosphorylation. And cytotoxicity of NU6102 after a 24 h exposure exhibits an LC50 of 2.6 μM in SKUT-1B cells.
Furthermore, NU6102 inhibits cell growth and causes cell cycle phase arrest in human breast cancer cell lines. Additionally, NU6102 results in G2/M arrest in asynchronously growing cell lines and G1/S arrest in cells released from serum starvation.
NU6102 selectively inhibits the growth of CDK2 WT (wild type) with a GI50 of 14 μM. However, NU6102 inhibits KO MEFs (knockout mouse embryo fibroblasts) growth with a GI50 >30 μM.
In Balb/C mice, NU6102 is liberated following either i.p. or i.v. administration of NU6301. And following i.v. administration NU6102 reaches its peak plasma levels of 12 μM after 5 mins. Whereas following administration of the maximum administrable dose of NU6102 i.v. achieves its peak concentration of 0.92 μM.
NU6102 and its prodrug NU6301 have pharmacological properties consistent with CDK2 inhibition. The N-acetyl prodrug of NU6102, NU6301, can rapidly convert to NU6102 and displays markedly improved aqueous solubility. In mice bearing SKUT-1B human tumor xenografts, 120 mg/kg NU6301 is administrated every 8 h or every 12 h for 10 days. Repeated dosing of NU6301 at 120 mg/kg for 10 days causes a significant increase in the median time for tumor volume to quadruple.
In conclusion, the CDK2 inhibitor NU6102 exhibits anti-cancer activity in vitro and in vivo. It facilitates the further evaluation of CDK2 as a target in cancer.
Reference:
[1]. Ian R Hardcastle, et al. J Med Chem. 2004 Jul 15;47(15):3710-22.
[2]. Huw D Thomas , et al. Eur J Cancer. 2011 Sep;47(13):2052-9.