PD-L1 is expressed in many types of cancers. And its high expression in tumor cells or presence in TME can be indicative of tumor-infiltrating lymphocytes (TILs).
Blockade of PD-1/PD-L1 can strengthen the function of effector T cells. Blockade of PD-1/PD-L1 can strengthen the function of effector T cells. Additionally, this blockade also increases T cells’ production of cytokines through reactivation.
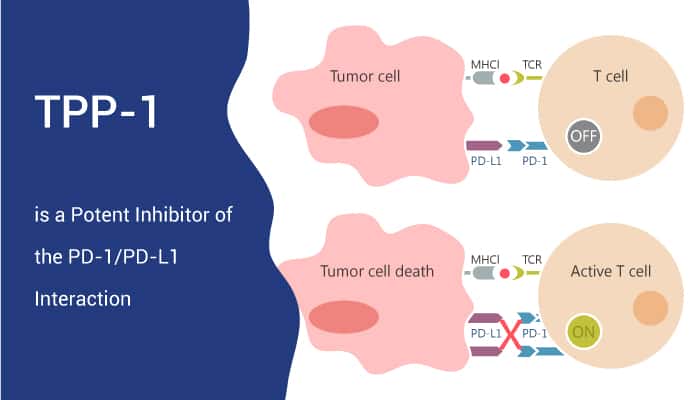
TPP-1 is a potent inhibitor of the PD-1/PD-L1 interaction.
TPP-1 binds to PD-L1 with high affinity and blocks PD-1/PD-L1 interaction. The KD value is a critical parameter to evaluate the binding affinity of two molecules. The KD value of PD-L1 with its natural binding ligand, PD-1, is about 500 nM. However, The KD value of PD-L1 with TPP-1 peptide is about 95 nmol/L. This value is around five times less than with PD-1. The binding site of TPP-1 to PD-L1 is close to the interactive site of PD-1 and PD-L1.
SPP-1 is a scrambled TPP-1 peptide and acts as a control.
Matured DCs has a high PD-L1 expression. CD4+ T cells are activated by CD3/CD28 antibody and IL-2. TPP-1 at a concentration of 20 μM significantly induces IFNγ release. TPP-1 (4 μM) reactivates T-cell functions, and significantly higher than the control and SPP-1 group. Furthermore, the TPP-1 group shows similar outcomes for cell proliferation.
Besides, in vivo, in a 5-6-week-old female Balb/c nude mice injected with H460 cells transfected with the plvx-puro/luciferase lentiviral vector. TPP-1 inhibits tumor growth when compares with the SPP-1 group. The growth rate in TPP-1-treated mice is 56%. But when administered in the absence of T cells (control group), TPP-1 has no effect on the growth of the H460-luc tumors. As a result, this means TPP-1 inhibits tumor growth in a xenograft model via reactivating T-cell function.
In conclusion, TPP-1 is a peptide inhibitor of PD-1/PD-L1 interaction. It could bind specifically to PD-L1 with high affinity and block PD-1/PD-L1 interaction. As a result, TPP-1 works as an inhibitory candidate for PD-L1-targeted cancer research.
Reference:
[1]. Chunlin Li, et al. Cancer Immunol Res. 2018 Feb;6(2):178-188.