KRAS gene is one of the most frequently mutated oncogenes in cancer. KRAS encodes a small, membrane-bound GTPase that relays signals from receptor tyrosine kinases (RTKs). It promotes cell proliferation, cell differentiation, or cell survival. In normal cells, KRAS functions as a molecular switch, cycling between an inactive, GDP-bound “off” state and an active, GTP-bound “on” state. Guanine nucleotide exchange factor (GEF) proteins tightly regulate this switch. They exchange GDP for GTP, and GTPase-activating proteins (GAPs), which enhance the intrinsically slow GTPase activity of KRAS. Somatic KRAS mutations attenuate the GAP-mediated enzymatic activity of the protein, resulting in accumulation of GTP-bound, active KRAS and hyperactivation of downstream signaling, which leads to uncontrolled cell proliferation. Mutant KRAS has remained a challenging therapeutic target given the scarcity of traditional druggable pockets on its surface. Fortunately, LC-2 is a potent and first-in-class PROTAC capable of degrading endogenous KRAS G12C.
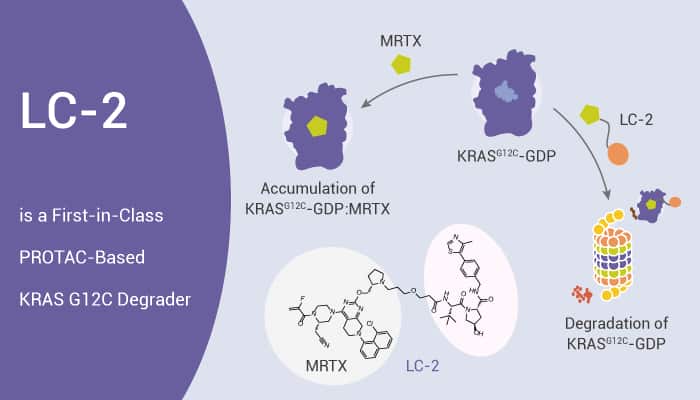
LC-2 is a potent and first-in-class PROTAC capable of degrading endogenous KRAS G12C. Meanwhile, it has DC50s between 0.25 and 0.76 μM. It covalently binds KRAS G12C with an MRTX849 warhead and recruits the E3 ligase VHL. It also displays rapid KRAS G12C degradation, leading to suppression of MAPK signaling in both homozygous and heterozygous KRAS G12C cell lines. Furthermore, it induces degradation of endogenous KRASG12C in multiple KRAS mutant cancer cells with DC50s between 0.25 and 0.76 μM. Moreover, LC-2 occurs via a bona fide PROTAC mechanism. In addition, LC-2 modulates Erk signaling in homozygous and heterozygous KRAS mutant cell lines.
In summary, LC-2 can serve as a tool compound to investigate biology in the context of rapid KRAS G12C degradation. It also opens new opportunities for targeting KRAS mutants in cancer therapy.
Reference:
Bond MJ, et al. ACS Cent Sci. 2020;6(8):1367-1375.