PARP is an enzyme that involved in a number of cellular processes such as DNA repair, and programmed cell death. The PARP family comprises 17 members. They have all very different structures and functions in the cell. Importantly, the main role is to detect and signal single-strand DNA breaks (SSB) to the enzymatic machinery involved in the SSB repair. In addition, PARP is composed of four domains: a DNA-binding domain, a caspase-cleaved domain, an auto-modification domain, and a catalytic domain. In the presence of damaged DNA, the DNA-binding domain will bind the DNA and induce a conformational shift. PARP inhibitors have the potential for ovarian cancer, breast cancer, pancreatic cancer and prostate cancer treatment. These tumors harbor germline or somatic BRCA1/2 mutations and/or homologous recombinant repair mutation.
Venadaparib (also known as IDX-1197) is a potent, selective and orally active PARP1/2 inhibitor with strong antitumor activities. By contrast, it is inactive against PARP5. In fact, Venadaparib prevents the repair of DNA single-strand breaks (SSB) and promotes the conversion of SSB to double-stranded breaks (DSB). In preclinical trials, in DNA damage-induced Hela cells, Venadaparib significantly inhibits PARP1-mediated PAR expression. While, Venadaparib also inhibits cell growth in human cancer cell lines. In the germline BRCA1-mutated ovarian cancer PDX (Patient-Derived tumor Xenograft) model, oral administration of Venadaparib exhibits significant PAR inhibition in tumor tissues. In addition, Venadaparib inhibits tumor growth in a dose-dependent manner. Moreover, Venadaparib also exhibits antitumor activity against breast cancer PDX models, which included both germline BRCA-mutated and non-germline BRCA-mutated models.
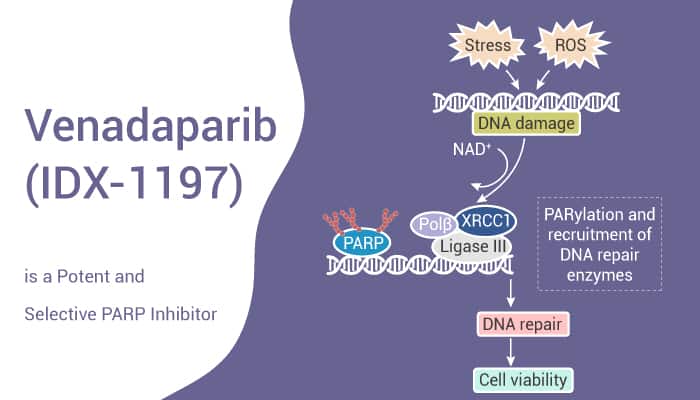
To sum up, Venadaparib is a highly selective and orally active PARP1/2 inhibitor with significant in vitro and in vivo activities in multiple cancer models.
References:
[1] Myongjae Lee, et al. American Association for Cancer Research, 2018.